Ambush Predator’ Guild – Are There Developmental Rules Underlying Body Shape Evolution in Ray-Finned Fishes? Erin E Maxwell1* and Laura AB Wilson2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Diversity of Fish (Pisces) in Freshwater
Hydrobiologia (2008) 595:545–567 DOI 10.1007/s10750-007-9034-0 FRESHWATER ANIMAL DIVERSITY ASSESSMENT Global diversity of fish (Pisces) in freshwater C. Le´veˆque Æ T. Oberdorff Æ D. Paugy Æ M. L. J. Stiassny Æ P. A. Tedesco Ó Springer Science+Business Media B.V. 2007 Abstract The precise number of extant fish spe- species live in lakes and rivers that cover only 1% cies remains to be determined. About 28,900 species of the earth’s surface, while the remaining 16,000 were listed in FishBase in 2005, but some experts species live in salt water covering a full 70%. While feel that the final total may be considerably higher. freshwater species belong to some 170 families (or Freshwater fishes comprise until now almost 13,000 207 if peripheral species are also considered), the species (and 2,513 genera) (including only fresh- bulk of species occur in a relatively few groups: water and strictly peripheral species), or about the Characiformes, Cypriniformes, Siluriformes, 15,000 if all species occurring from fresh to and Gymnotiformes, the Perciformes (noteably the brackishwaters are included. Noteworthy is the fact family Cichlidae), and the Cyprinodontiformes. that the estimated 13,000 strictly freshwater fish Biogeographically the distribution of strictly fresh- water species and genera are, respectively 4,035 species (705 genera) in the Neotropical region, 2,938 (390 genera) in the Afrotropical, 2,345 (440 Guest editors: E. V. Balian, C. Le´veˆque, H. Segers & K. Martens genera) in the Oriental, 1,844 (380 genera) in the Freshwater Animal Diversity Assessment Palaearctic, 1,411 (298 genera) in the Nearctic, and 261 (94 genera) in the Australian. -

Article Evolutionary Dynamics of the OR Gene Repertoire in Teleost Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Article Evolutionary dynamics of the OR gene repertoire in teleost fishes: evidence of an association with changes in olfactory epithelium shape Maxime Policarpo1, Katherine E Bemis2, James C Tyler3, Cushla J Metcalfe4, Patrick Laurenti5, Jean-Christophe Sandoz1, Sylvie Rétaux6 and Didier Casane*,1,7 1 Université Paris-Saclay, CNRS, IRD, UMR Évolution, Génomes, Comportement et Écologie, 91198, Gif-sur-Yvette, France. 2 NOAA National Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. 3Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20560, U.S.A. 4 Independent Researcher, PO Box 21, Nambour QLD 4560, Australia. 5 Université de Paris, Laboratoire Interdisciplinaire des Energies de Demain, Paris, France 6 Université Paris-Saclay, CNRS, Institut des Neurosciences Paris-Saclay, 91190, Gif-sur- Yvette, France. 7 Université de Paris, UFR Sciences du Vivant, F-75013 Paris, France. * Corresponding author: e-mail: [email protected]. !1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Teleost fishes perceive their environment through a range of sensory modalities, among which olfaction often plays an important role. -

JVP 26(3) September 2006—ABSTRACTS
Neoceti Symposium, Saturday 8:45 acid-prepared osteolepiforms Medoevia and Gogonasus has offered strong support for BODY SIZE AND CRYPTIC TROPHIC SEPARATION OF GENERALIZED Jarvik’s interpretation, but Eusthenopteron itself has not been reexamined in detail. PIERCE-FEEDING CETACEANS: THE ROLE OF FEEDING DIVERSITY DUR- Uncertainty has persisted about the relationship between the large endoskeletal “fenestra ING THE RISE OF THE NEOCETI endochoanalis” and the apparently much smaller choana, and about the occlusion of upper ADAM, Peter, Univ. of California, Los Angeles, Los Angeles, CA; JETT, Kristin, Univ. of and lower jaw fangs relative to the choana. California, Davis, Davis, CA; OLSON, Joshua, Univ. of California, Los Angeles, Los A CT scan investigation of a large skull of Eusthenopteron, carried out in collaboration Angeles, CA with University of Texas and Parc de Miguasha, offers an opportunity to image and digital- Marine mammals with homodont dentition and relatively little specialization of the feeding ly “dissect” a complete three-dimensional snout region. We find that a choana is indeed apparatus are often categorized as generalist eaters of squid and fish. However, analyses of present, somewhat narrower but otherwise similar to that described by Jarvik. It does not many modern ecosystems reveal the importance of body size in determining trophic parti- receive the anterior coronoid fang, which bites mesial to the edge of the dermopalatine and tioning and diversity among predators. We established relationships between body sizes of is received by a pit in that bone. The fenestra endochoanalis is partly floored by the vomer extant cetaceans and their prey in order to infer prey size and potential trophic separation of and the dermopalatine, restricting the choana to the lateral part of the fenestra. -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Amorphometric and Meristic Study of the Halfbeak, Hyporhamphus
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1993 Amorphometric and Meristic Study of the Halfbeak, Hyporhamphus unifasciatus (Teleostei: Hemiramphidae) from the Western Atlantic, with the Description of a New Species Heidi M. Banford College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Marine Biology Commons, and the Oceanography Commons Recommended Citation Banford, Heidi M., "Amorphometric and Meristic Study of the Halfbeak, Hyporhamphus unifasciatus (Teleostei: Hemiramphidae) from the Western Atlantic, with the Description of a New Species" (1993). Dissertations, Theses, and Masters Projects. Paper 1539617658. https://dx.doi.org/doi:10.25773/v5-pbsc-sy52 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. A MORPHOMETRIC AND MERISTIC STUDY OF THE HALFBEAK, HYPORHAMPHUS UNIFASCIATUS (TELEOSTEI: HEMIRAMPHIDAE) FROM THE WESTERN ATLANTIC, WITH THE DESCRIPTION OF A NEW SPECIES A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts by Heidi M. Banford 1993 This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts Heidi M. Banford Approved, July 1993 Jojm A. Musick,' Ph.D. flmittee Chairman/Advisor ~ t M . ^ Herbert M. Austin, Ph.D. -

THE FAMILY SYNGNATHIDAE (PISCES: SYNGNATHIFORMES) of Taiwanl
Bull. Inst. Zool., Academia Sinica 22(1): 67-82 (1983) THE FAMILY SYNGNATHIDAE (PISCES: SYNGNATHIFORMES) OF TAIWANl SIN-CHE LEE Institlile of Zoology, Academia Sinica, Taipei, Taiwan lI5t Republic of China (Received October 12, 1982) Sin-Che Lee (1983) The family Syngnathidae (Pisces: Syngnathiformes) of Tai wan. Bull. Inst. Zool., Academia Sinica 22(1): 67-82. A systematic review of the syn gnathid fishes found in the waters of Taiwan and its adjacent islands documents a total of 22 species in 16 genera. Among them,. Dunckerocampus dactyliophorus, Coelo notus liaspis, Halicampus koilomatodon, Microphis manadensis, Hippichthys heptagon us, H. spidfer, Syngnathus pelagicus, Corythoichthys f!avofasdatus, Solegnathus hardwickii, Haliichthys taeniophorous and Hippocampus erinaceus are new records for the Taiwan area. A family diagnosis. key to genera and species, brief synonyms, diagnosis, remarks and illustrations of each species are given. trimaculatus and both H. kelloggi and H. atteri The syngnathids including the pipefishes mus are synonyms of H. kuda. Solegnathus and seahorses are small fishes of tropical and guntheri and Syngnathus argyristictus are pro moderately warm temperate shallow coastal visionally removed from the list since no· data waters. Seahorses are more highly specialized are available. Thus only 8 valid species are than pipefishes but most seahorses are confined remained in Chen's list of Taiwan syngnathids. to marine waters and restricted to particular Neverthless, after a period of intensive collec habitat. On the other hand pipefishes have a tion the present author has found 13 addi wider distribution; they can tolerate greater fional species making a total 21 syngnathid temperature and salinity ranges. -

Leo Semenovich Berg and the Biology of Acipenseriformes: a Dedication
Environmental Biology of Fishes 48: 15–22, 1997. 1997 Kluwer Academic Publishers. Printed in the Netherlands. Leo Semenovich Berg and the biology of Acipenseriformes: a dedication Vadim J. Birstein1 & William E. Bemis2 1 The Sturgeon Society, 331 West 57th Street, Suite 159, New York, NY 10019, U.S.A. 2 Department of Biology and Graduate Program in Organismic and Evolutionary Biology, University of Massachusetts, Amherst, MA 01003, U.S.A. Received 5.3.1996 Accepted 23.5.1996 Key words: T. Dobzhansky, A. Sewertzoff, T. Lysenko, Paleonisciformes, biogeography This volume is dedicated to the memory of Leo Semenovich Berg (1876–1950), a Russian ichthyologist and geographer. In the foreword to the English translation of Berg’s remarkable treatise, ‘Nomogenesis or evolu- tion according to law’, Theodosius Dobzhansky wrote: ‘Berg was one of the outstanding intellects among Russian scientists. The breadth of his interests and the depth as well as the amplitude of his scholarship were remarkable. He had the reputation of being a ‘walking library’, because of the amount of information he could produce from his memory’ (Dobzhansky 1969, p. xi). Berg was prolific, publishing 217 papers and monographs on ichthyology, 30 papers on general zoology and biology, 20 papers on paleontology, 32 papers on zoogeo- graphy, 320 papers and monographs on geography, geology, and ethnography, as well as 290 biographies, obituaries, and popular articles (Berg 1955, Sokolov 1955). Berg was born 120 years ago, on 14 March 1876, in Sciences. Berg was never formally recognized by the town of Bendery. According to laws of the Rus- the Soviet Academy for his accomplishments in sian Empire, Berg could not enter the university as biology, and only later (1946) was he elected a mem- a Jew, so he was baptized and became a Lutheran, ber of the Geography Branch of the Soviet Acade- which allowed him to study and receive his diploma my of Sciences (Figure 1). -
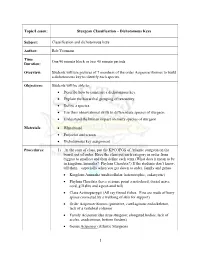
Sturgeon Classification – Dichotomous Keys Subject
Topic/Lesson: Sturgeon Classification – Dichotomous Keys Subject: Classification and dichotomous keys Author: Rob Yeomans Time One 90 minute block or two 45 minute periods Duration: Overview: Students will use pictures of 7 members of the order Acipenseriformes to build a dichotomous key to identify each species. Objectives: Students will be able to: • Describe how to construct a dichotomous key. • Explain the hierarchal grouping of taxonomy. • Define a species. • Use their observational skills to differentiate species of sturgeon. • Understand the human impact on many species of sturgeon. Materials: • Whiteboard • Projector and screen • Dichotomous key assignment Procedures: 1) At the start of class, put the KPCOFGS of Atlantic sturgeon on the board, out of order. Have the class put each category in order from biggest to smallest and then define each term (What does it mean to be in kingdom Animalia? Phylum Chordata?) If the students don’t know, tell them—especially when you get down to order, family and genus. • Kingdom Animalia (multicellular, heterotrophic, eukaryotic) • Phylum Chordata (have at some point a notochord, dorsal nerve cord, gill slits and a post-anal tail) • Class Actinopterygii (All ray finned fishes. Fins are made of bony spines connected by a webbing of skin for support) • Order Acipenseriformes (primitive, cartilaginous endoskeleton, lack of a vertebral column) • Family Acipenseridae (true sturgeon; elongated bodies, lack of scales, anadromous, bottom feeders) • Genus Acipenser (Atlantic Sturgeon) 1 • Species oxyrinchus Comment to the class that there are actually two subspecies of oxyrinchus. Acipenser oxyrinchus oxyrinchus is the Atlantic sturgeon and Acipenser oxyrinchus desotoi is the Gulf sturgeon 2) Reinforce the fact that sturgeon are a primitive fish and fossils have been found dating back 144-65 million years ago. -

Downloaded for Five Nuclear Genes 243 (KIAA1239, MYH6, RIPK4, RAG1, SH3PX3), and Four Mitochondrial Genes (12S, 16S, 244 COX1 and CYTB)
bioRxiv preprint doi: https://doi.org/10.1101/247304; this version posted March 26, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 2 How the Central American Seaway and 3 an ancient northern passage affected 4 flatfish diversification 5 6 Lisa Byrne1, François Chapleau1, and Stéphane Aris-Brosou*,1,2 7 8 1Department of Biology, University of Ottawa, Ottawa, ON, CANADA 9 2Department of Mathematics & Statistics, University of Ottawa, Ottawa, ON, CANADA 10 11 *Corresponding author: E-mail: [email protected] 12 1 bioRxiv preprint doi: https://doi.org/10.1101/247304; this version posted March 26, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 13 Abstract 14 While the natural history of flatfish has been debated for decades, the mode of 15 diversification of this biologically and economically important group has never been 16 elucidated. To address this question, we assembled the largest molecular data set to date, 17 covering > 300 species (out of ca. 800 extant), from 13 of the 14 known families over 18 nine genes, and employed relaxed molecular clocks to uncover their patterns of 19 diversification. As the fossil record of flatfish is contentious, we used sister species 20 distributed on both sides of the American continent to calibrate clock models based on 21 the closure of the Central American Seaway (CAS), and on their current species range. -

5. the Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and Other Vertebrates
Rendiconti della Società Paleontologica Italiana, 4, 2014, pp. 37-63 Excursion guidebook CBEP 2014-EPPC 2014-EAVP 2014-Taphos 2014 Conferences The Bolca Fossil-Lagerstätten: A window into the Eocene World (editors C.A. Papazzoni, L. Giusberti, G. Carnevale, G. Roghi, D. Bassi & R. Zorzin) 5. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates [ CARNEVALE, } F. BANNIKOV, [ MARRAMÀ, ^ C. TYLER & ? ZORZIN G. Carnevale, Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, I-10125 Torino, Italy; [email protected] A.F. Bannikov, Borisyak Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya 123, Moscow 117997, Russia; [email protected] G. Marramà, Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso, 35 I-10125 Torino, Italy; [email protected] J.C. Tyler, National Museum of Natural History, Smithsonian Institution (MRC-159), Washington, D.C. 20560 USA; [email protected] R. Zorzin, Sezione di Geologia e Paleontologia, Museo Civico di Storia Naturale di Verona, Lungadige Porta Vittoria 9, I-37129 Verona, Italy; [email protected] INTRODUCTION ][` ~[~ `[ =5} =!+~ [=5~5 Ceratoichthys pinnatiformis5 #] ~}==5[ ~== }}=OP[~` [ "O**""P "}[~* "+5$!+? 5`=5` ~]!5`5 =5=[~5_ O"!P#! [=~=55~5 `#~! ![[[~= O"]!#P5`` `5} 37 G. Carnevale, A.F. Bannikov, G. Marramà, J.C. Tyler & R. Zorzin FIG. 1_Ceratoichthys pinnatiformis~=5"!Q5=` 5. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates `== `]5"`5`" O*!P[~ `= =5<=[ ~#_5` [#5!="[ [~OQ5=5""="P5 ` [~`}= =5^^+55 ]"5++"5"5* *5 [=5` _5 [==5 *5]5[=[[5* [5=~[` +~++5~5=!5 ["5#+?5?5[=~[+" `[+=\`` 5`55`_= [~===5[=[5 ```_`5 [~5+~++5 [}5` `=5} 5= [~5O# "~++[=[+ P5`5 ~[O#P #"5[+~` [=Q5 5" QRQ5$5 ][5**~= [`OQ= RP`=5[` `+5=+5`=` +5 _O# P5+5 O? ]P _ #`[5[=~ [+#+?5` !5+`}==~ `5``= "!=Q5 "`O? ]P+5 _5`~[ =`5= G. -

Osteichthyes, Actinopterygii) from the Early Triassic of Northwestern Madagascar
Rivista Italiana di Paleontologia e Stratigrafia (Research in Paleontology and Stratigraphy) vol. 123(2): 219-242. July 2017 REDESCRIPTION OF ‘PERLEIDUS’ (OSTEICHTHYES, ACTINOPTERYGII) FROM THE EARLY TRIASSIC OF NORTHWESTERN MADAGASCAR GIUSEPPE MARRAMÀ1*, CRISTINA LOMBARDO2, ANDREA TINTORI2 & GIORGIO CARNEVALE3 1*Corresponding author. Department of Paleontology, University of Vienna, Geozentrum, Althanstrasse 14, 1090 Vienna, Austria. E-mail: [email protected] 2Dipartimento di Scienze della Terra, Università degli Studi di Milano, Via Mangiagalli 34, I-20133 Milano, Italy. E-mail: cristina.lombardo@ unimi.it; [email protected] 3Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, I-10125 Torino, Italy. E-mail: giorgio.carnevale@ unito.it To cite this article: Marramà G., Lombardo C., Tintori A. & Carnevale G. (2017) - Redescription of ‘Perleidus’ (Osteichthyes, Actinopterygii) from the Early Triassic of northwestern Madagascar . Riv. It. Paleontol. Strat., 123(2): 219-242. Keywords: Teffichthys gen. n.; TEFF; Ankitokazo basin; geometric morphometrics; intraspecific variation; basal actinopterygians. Abstract. The revision of the material from the Lower Triassic fossil-bearing-nodule levels from northwe- stern Madagascar supports the assumption that the genus Perleidus De Alessandri, 1910 is not present in the Early Triassic. In the past, the presence of this genus has been reported in the Early Triassic of Angola, Canada, China, Greenland, Madagascar and Spitsbergen. More recently, it has been pointed out that these taxa may not be ascri- bed to Perleidus owing to several anatomical differences. The morphometric, meristic and morphological analyses revealed a remarkable ontogenetic and individual intraspecific variation among dozens of specimens from the lower Triassic of Ankitokazo basin, northwestern Madagascar and allowed to consider the two Malagasyan species P.