Effects of Pratylenchus Vulnus on the Growth of Sour Orange R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influence of Pratylenchus Vulnus and Meloidogyne Hapla on the Growth of Rootstocks of Rose~ G
influence of Pratylenchus vulnus and Meloidogyne hapla on the Growth of Rootstocks of Rose~ G. S. SANTO 2 and BERT LEAR '~ Abstract: Pratylenchus vulnus is involved in a disease of Rosa noisettiana 'Manetti" rose rootstock characterized by darkening of roots, death of feeder toots, and stunting of entire plants. The disease is more severe when plants are grown in silt loam soil than when they are grown in sandy loam soil. The nematodes reproduce best in silt loam soil at 20 C. Meloidogyne hapla did not affect the growth of Manetti. Rosa sp. 'Dr. Huey', Manetti, and R. odorata rose rootstocks were found to be good hosts for P. vulnus whereas R. multiflora was less suitable. M. hapla re- produced well on R. odorata, Dr. Huey, and R. multi[lora, but not on Maneni. Key Words: root- lesion nematode, root-knot nematode, reproduction, soil temperature, soil type. Pratylenchus vulnus Allen and Jensen roses was associated with a reduction in the was first reported from rose roots in Cali- population of M. hapla and Xiphinema fornia in 1953 (14). A 1970 survey of com- arnericanum Cobb as a result of multiple mercial rose greenhouses in northern Cali- applications of 1,2-dibromo-3-chloropro- fornia shows that this nematode is now pane (DBCP) (8). widely distributed (9), and Allen and Jen- Rosa noisettiana Thory 'Manetti' is the sen (l) also report that P. vulnus is widely most popular rootstock used in growing distributed on field-grown roses in southern greenhouse roses for cut flowers. R. odorata California. Sweet and Rosa sp. -

ENTO-364 (Introducto
K. K. COLLEGE OF AGRICULTURE, NASHIK DEPARTMENT OF AGRICULTURAL ENTOMOLOGY THEORY NOTES Course No.:- ENTO-364 Course Title: - Introductory Nematology Credits: - 2 (1+1) Compiled By Prof. T. B. Ugale & Prof. A. S. Mochi Assistant Professor Department of Agricultural Entomology 0 Complied by Prof. T. B. Ugale & Prof. A. S. Mochi (K. K. Wagh College of Agriculture, Nashik) TEACHING SCHEDULE Semester : VI Course No. : ENTO-364 Course Title : Introductory Nematology Credits : 2(1+1) Lecture Topics Rating No. 1 Introduction- History of phytonematology and economic 4 importance. 2 General characteristics of plant parasitic nematodes. 2 3 Nematode- General morphology and biology. 4 4 Classification of nematode up to family level with 4 emphasis on group of containing economical importance genera (Taxonomic). 5 Classification of nematode by habitat. 2 6 Identification of economically important plant nematodes 4 up to generic level with the help of key and description. 7 Symptoms caused by nematodes with examples. 4 8 Interaction of nematodes with microorganism 4 9 Different methods of nematode management. 4 10 Cultural methods 4 11 Physical methods 2 12 Biological methods 4 13 Chemical methods 2 14 Entomophilic nematodes- Species Biology 2 15 Mode of action 2 16 Mass production techniques for EPN 2 Reference Books: 1) A Text Book of Plant Nematology – K. D. Upadhay & Kusum Dwivedi, Aman Publishing House 2) Fundamentals of Plant Nematology – E. J. Jonathan, S. Kumar, K. Deviranjan, G. Rajendran, Devi Publications, 8, Couvery Nagar, Karumanolapam, Trichirappalli, 620 001. 3) Plant Nematodes - Methodology, Morphology, Systematics, Biology & Ecology Majeebur Rahman Khan, Department of Plant Protection, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh, India. -

Silencing Parasitism Effectors of the Root Lesion Nematode, Pratylenchus Thornei
Silencing parasitism effectors of the root lesion nematode, Pratylenchus thornei. This thesis is presented for the degree of Doctor of Philosophy of Murdoch University by Sameer Dilip Khot B.Sc. (Botany) & M.Sc. (Plant Pathology & Mycology), University of Mumbai, India M.S. (Plant Pathology), North Dakota State University, USA Western Australian State Agricultural Biotechnology Centre School of Veterinary and Life Sciences Murdoch University Perth, Western Australia 2018 1 DECLARATION I declare that this thesis is my own account of my research and contains as its main content work which has not previously been submitted for a degree at any tertiary education institution. Signature: Sameer D. Khot Date: 22-01-2018 2 ABSTRACT The root lesion nematode (RLN), Pratylenchus thornei, is a biotrophic migratory pest of plant roots and its infestation causes losses in many economically important crops. RNA interference (RNAi) is a naturally occurring eukaryotic phenomenon and can be used to silence parasitism effector genes of P. thornei using host-mediated RNAi. This may be developed as an environmentally friendly and a cost-effective control strategy. The overall aims of this research were to investigate the effects of in vitro and in planta RNAi silencing of putative P. thornei parasitism effector genes, and their nematicidal effects in two host plants. Five putative target parasitism genes vital for nematode entry into roots (Pt-Eng-1, Pt- PL), feeding (Pt-CLP) and suppressing host defence responses (Pt-UEP, Pt-GST) were identified, validated in silico using comparative bioinformatics, cloned into suitable in vitro transcription and binary vectors, and advanced to RNAi studies. -

Diversity, Phylogeny, Characterization and Diagnostics of Root-Knot and Lesion Nematodes
Diversity, phylogeny, characterization and diagnostics of root-knot and lesion nematodes Toon Janssen Promotors: Prof. Dr. Wim Bert Prof. Dr. Gerrit Karssen Thesis submitted to obtain the degree of doctor in Sciences, Biology Proefschrift voorgelegd tot het bekomen van de graad van doctor in de Wetenschappen, Biologie 1 Table of contents Acknowledgements Chapter 1: general introduction 1 Organisms under study: plant-parasitic nematodes .................................................... 11 1.1 Pratylenchus: root-lesion nematodes ..................................................................................... 13 1.2 Meloidogyne: root-knot nematodes ....................................................................................... 15 2 Economic importance ..................................................................................................... 17 3 Identification of plant-parasitic nematodes .................................................................. 19 4 Variability in reproduction strategies and genome evolution ..................................... 22 5 Aims .................................................................................................................................. 24 6 Outline of this study ........................................................................................................ 25 Chapter 2: Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. 1 Abstract -

A Reappraisal of Tylenchina (Nemata) 1
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1987 A Reappraisal of Tylenchina (Nemata) 1. For a New Approach to the Taxonomy of Tylenchina Michel Luc Muséun1 national d'Histoire naturelle Armand R. Maggenti University of California - Davis Renaud Fortuner California Department of Food and Agriculture Dewey J. Raski University of California - Davis Etienne Geraert Instituut voor Dierkunde Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Luc, Michel; Maggenti, Armand R.; Fortuner, Renaud; Raski, Dewey J.; and Geraert, Etienne, "A Reappraisal of Tylenchina (Nemata) 1. For a New Approach to the Taxonomy of Tylenchina" (1987). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 109. https://digitalcommons.unl.edu/parasitologyfacpubs/109 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Tribune A REAPPRAISAL OF "IYL,ENCHINA (NEMATA) 1. FOR A NEW APPROACH TO THE TAXONOMY OF TYLENCHINA Michel LUC",Armand R. MAGGENTI**, Renaud FORTUNER***, Dewey J. RASKI** and Etienne GERAERT**** * Muséun1 national d'Histoire naturelle, Laboratoire des Vers, 61, rue de Buffon, 75005 Paris; ** Division of Nematology, University of California, Davis, CA 95616, USA; *** California Department of Food and Agriculture, Analysis and Identification (Nematology), 1220 N. Street, Sacramento, CA 95814, USA, and **** Rijksuniversiteit Gent, Instituut voor Dierkunde, Ledeganckstraat 35, 9000 Gent, Belgium. -

Sudan University of Science and Technology College of Graduate
Sudan University of Science and Technology College of Graduate Studies Studies on Plant Parasitic Nematodes on Banana in Sennar and Kassala States دراﺳﺎت ﻋﻠﻰ اﻟﻨﯿﻤﺎﺗﻮدا اﻟﻤﺘﻄﻔﻠﺔ ﻋﻠﻰ ﻧﺒﺎت اﻟﻤﻮز ﻓﻰ وﻻﯾﺘﻰ ﺳﻨﺎر وﻛﺴﻼ A thesis submitted in to the Sudan University of Science and Technology in Fulfillment of the requirement for M.Sc. (Agric) in crop protection (Plant Pathology) By Duria Abdelsalam Eltahir Elshakh Supervisor: Dr. Ibrahim Saeed Mohammed 2014 ﺑﺳم ﷲ اﻟرﺣﻣن اﻟرﺣﯾم Sudan University of Science and Technology College of Graduate Studies Studies on Plant Parasitic Nematodes on Banana in Sennar and Kassala States دراﺳﺎت ﻋﻠﻰ اﻟﻨﯿﻤﺎﺗﻮدا اﻟﻤﺘﻄﻔﻠﺔ ﻋﻠﻰ ﻧﺒﺎت اﻟﻤﻮز ﻓﻰ وﻻﯾﺘﻰ ﺳﻨﺎر وﻛﺴﻼ A thesis submitted in the requirement of the Degree of M.Sc. Agric. In Crop Protection (Plant Pathology) By Duria Abdelsalam Eltahir Elshakh B.Sc. in Crop Protection University of Zagazig, Egypt(1986) Main Supervisor: Dr. Ibrahim Saeed Mohammed Co. Supervisor: Prof. Gamal Abdalla Elbadri 2014 Dedication To the soul of my father Lovely mother Intimate husband Brothers and sisters To all those who search of knowledge 3 ACKNOWLEDGMENT Thanks and praise to the Beneficent and Merciful God who provided me with health, strength and patience to have this drop from the renewable flood of knowledge. I am gratefully to my supervisors, Doctor Ibrahim Saeed Mohammed and Professor Gamal Abdalla Elbadri for all the encouragement, helpful guidance and continued support. Special thankS to my Co. supervisor Professor. Gamal Abdalla Elbadri under who's the supervision of work has been successfully carried out with his invaluable advices, unlimited helpful and for this correction of this study. -
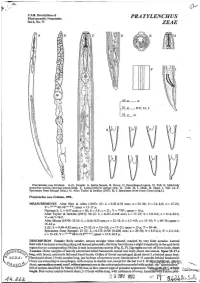
Pratylenchus Zeae
/p C.I.H. Descriptions of Plant-parasitic Nematodes PRATYLENCHUS Set 6, No. 77 ZEAE ' A D F V L 50 P A i5 ,B-F,H,I 25P ,G G Prutylenchus zeue Graham. A-G. Female. A. Entire female. B. Ovary. C. Oesophageal region. D. Tail. E. Mid-body ' transverse section showing lateral fields. F. Lateral field in surface view. G. Tails. H, I. Male. H. Head. I. Tail. (A-F. Specimens from Senegal (orig.). G. After Taylor & Jenkins (1957). H, I. Specimens from Ivory Coast (orig.).) PratyZemhus zeae Graham, 1951. MEASUREMENTS After Sher & Allen (1953): 99: L = 0.36-0.58 mm; a = 25-30; b = 5.4-8.0; c= 17-21; V = 26-43 68-763.4-6."; spear = 15-17 p. Neotype 9: L = 0.47 mm; a = 26; b = 5.9; c = 21 ; V = 30704;spear = 16 p. After Taylor & Jenkins (1957): 90 99: L = 0.413-0.639 mm; a = 17-25; b = 5.0-9.6; c = 11.2-24.1; v = 64.7-74.9. After Memy (1970): 25 99: L = 0.34-0.55 mm; a = 22-33; b = 3.3-4.9; c = 13-18; V = 69-74; spear = 15-18 p. 5 88: L = 0.40-0.42 mm; a = 27-32; b = 3.6-5.0; c = 17-21 ;spear = 15 p; T = 30-44. Specimens from Senegal: 25 99: L = 0.373-0.506 (0.428) mm; a = 20-30; b = 4.9-6.1 ; b' = 3.2-4.6; c = 15-19; V = 23-38 68.6-73.93-86.7; spear= 15.5-16.5 p. -

Genus Pratylenchus Filipjev: Multientry and Monoentry Keys and Diagnostic Relationships (Nematoda: Tylenchida: Pratylenchidae)
© Zoological Institute, St.Petersburg, 2002 Genus Pratylenchus Filipjev: multientry and monoentry keys and diagnostic relationships (Nematoda: Tylenchida: Pratylenchidae) A.Y. Ryss Ryss, A.Y. 2002. Genus Pratylenchus Filipjev: multientry and monoentry keys and diag- nostic relationships (Nematoda: Tylenchida: Pratylenchidae). Zoosystematica Rossica, 10(2), 2001: 241-255. Tabular (multientry) key to Pratylenchus is presented, and functioning of the computer- ized multientry image-operating key developed on the basis of the stepwise computer diagnostic system BIKEY-PICKEY is described. Monoentry key to Pratylenchus is given, and diagnostic relationships are analysed with the routine taxonomic methods as well as with the use of BIKEY diagnostic system and by the cluster tree analysis using STATISTICA program package. The synonymy Pratylenchus scribneri Steiner in Sherbakoff & Stanley, 1943 = P. jordanensis Hashim, 1983, syn. n. is established. Con- clusion on the transition from amphimixis to parthenogenesis as one of the leading evolu- tionary factors for Pratylenchus is drawn. A.Y. Ryss, Zoological Institute, Russian Academy of Sciences, Universitetskaya nab. 1, St.Petersburg 199034, Russia. Identification of nematode species is difficult diagnostic system, and by the cluster tree analy- because of relative poverty and significant sis using STATISTICA program package intraspecific variability of diagnostic characters. (STATISTICA, 1995). The genus Pratylenchus Filipjev is an example of a group with large number of species (49 valid Material and the basic information sources species, more than 100 original descriptions) and complicated diagnostics. The genus has a world- The collections of the following institutions wide distribution and economic importance as were used in research: Zoological Institute, Rus- its species are the dangerous parasites of agri- sian Academy of Sciences; Institute for Nema- cultural crops. -

193 Molecular, Morphological and Thermal Characters of 19 Pratylenchus Spp. and Relatives Using the D3 Segment of the Nuclear Ls
MOLECULAR, MORPHOLOGICAL AND THERMAL CHARACTERS OF 19 PRATYLENCHUS SPP. AND RELATIVES USING THE D3 SEGMENT OF THE NUCLEAR LSU rRNA GENE Lynn K. Carta, Andrea M. Skantar, and Zafar A. Handoo USDA-ARS, Plant Sciences Institute, Nematology Lab, Beltsville, MD 20705, U.S.A. ABSTRACT Carta, L. K., A. M. Skantar, and Z. A. Handoo. 2001. Molecular, morphological and thermal charac- ters of 19 Pratylenchus spp. and relatives using the D3 segment of the nuclear LSU rRNA gene. Nem- atropica 31:195-209. Gene sequences are provided for the D3 segment of the large subunit rRNA gene in Pratylenchus agilis, P. hexincisus, P. teres, and P. zeae. They were aligned with the closest comparable previously pub- lished molecular sequences and evaluated with parsimony, distance and maximum-likelihood meth- ods. Different outgroups and more taxa in this study compared to a previous D3 tree resulted in improved phylogenetic resolution. Congruence of trees with thermal, vulval and lip characters was evaluated. A tropical clade of Pratylenchus with 2 lip annules was seen in all trees. Maximum-Parsimony and Quartet-Puzzling Maximum-Likelihood trees, with ambiguously-alignable positions excluded and Radopholus similis as an outgroup, had topologies congruent with species possessing 2, 3 or 4 lip annules. An updated sequence for Pratylenchus hexincisus indicated it was an outgroup of P. penetrans, P. arlingtoni, P. fallax and P. convallariae. Pratylenchus zeae was related to P. neglectus in a Neighbor-Join- ing tree, but was equivocal in others. The relatives of P. teres were P. neglectus and Hirschmanniella belli rather than morphometrically similar P. crenatus. -

Investigation on Iranian Pratylenchus Vulnus Populations by Morphological and Molecular Marker (RAPD- PCR)
Journal of Agricultural Technology 2012 Vol. 8(1): 219-231 Available online http://www.ijat-aatsea.com Journal of Agricultural Technology 2012, Vol. 8(1): 219-231 ISSN 1686-9141 Investigation on Iranian Pratylenchus vulnus populations by morphological and molecular marker (RAPD- PCR) Mansoureh Bakooie 1, Ebrahim Pourjam1*, and Mokhtar Jalali Javaran 2 1Department of Plant Entomology, School of Agriculture, Tarbiat Modares University; Tehran, Iran 2Department of Plant Breeding, School of Agriculture, Tarbiat Modares University; Tehran, Iran Mansoureh Bakooie, Ebrahim Pourjam and Mokhtar Jalali Javaran (2012) Investigation on Iranian Pratylenchus vulnus populations by morphological and molecular marker (RAPD- PCR). Journal of Agricultural Technology 8(1): 219-231. Morphological characteristics and genetic variability of two isolates of root-lesion nematode Pratylenchus vulnus, collected from the rhizospher of apple trees in Moghan and from maple trees in Behshar area’s, were analyzed. Morphological studies of cultured population on carrot disks showed that there are minor differences between two isolates. In both isolates, spermatheca are oval, relatively narrow and oval to round, head with three or four annules and in some specimens with three annules on one side and four annulus on the other side of the head. Tail shape was variable. Scanning electron microscopy (SEM) showed no considerable morphological difference of head in the two isolates. The investigation of genetic structure with RAPD-PCR marker indicated that two isolates have relatively high genetic variability (45%). The results of this research demonstrated morphometric similarity and genetic variability in the Iranian isolates of P. vulnus. This variability was not enough to allow us to divide them into two different species. -

Studies on the Morphology and Bio-Ecology of Nematode Fauna of Rewa
STUDIES ON THE MORPHOLOGY AND BIO-ECOLOGY OF NEMATODE FAUNA OF REWA A TMESIS I SUBMITTED FOR THE DEGREE OF DOCTOR OF PHlLOSOPHy IN ZOOLOGY A. P. S. UNIVERSITY. REWA (M. P.) INDIA 1995 MY MANOJ KUMAR SINGH ZOOLOGICAL RESEARCH LAB GOVT. AUTONOMOUS MODEL SCIENCE COLLEGE REWA (M. P.) INDIA La u 4 # s^ ' T5642 - 7 OCT 2002 ^ Dr. C. B. Singh Department of Zoology M Sc, PhD Govt Model Science Coll Professor & Head Rewa(M P ) - 486 001 Ref Date 3^ '^-f^- ^'^ir CERTIFICATE Shri Manoj Kumar Singh, Research Scholar, Department of Zoology, Govt. Model Science College, Rewa has duly completed this thesis entitled "STUDIES ON THE MORPHOLOGY AND BIO-ECOLOGY OF NEMATODE FAUNA OF REWA" under my supervision and guidance He was registered for the degree of Philosophy in Zoology on Jan 11, 1993. Certified that - 1. The thesis embodies the work of the candidate himself 2. The candidate worked under my guidance for the period specified b\ A. P. S. University, Rewa. 3. The work is upto the standard, both from, itscontentsas well as literary presentation point of view. I feel pleasure in commendingthis work to university for the awaid of the degree. (Dr. Co. Singh) or^ra Guide Professor & Head of Zoology department Govt. Model Science College (Autonomous) Rewa (M.P.) DECLARATION The work embodied in this thesis is original and was conducted druing the peirod for Jan. 1993 to July 1995 at the Zoological Research Lab, Govt. Model Science College Rewa, (M.P.) to fulfil the requirement for the degree of Doctor of Philosophy in Zoology from A.P.S. -

Nematology Training Manual
NIESA Training Manual NEMATOLOGY TRAINING MANUAL FUNDED BY NIESA and UNIVERSITY OF NAIROBI, CROP PROTECTION DEPARTMENT CONTRIBUTORS: J. Kimenju, Z. Sibanda, H. Talwana and W. Wanjohi 1 NIESA Training Manual CHAPTER 1 TECHNIQUES FOR NEMATODE DIAGNOSIS AND HANDLING Herbert A. L. Talwana Department of Crop Science, Makerere University P. O. Box 7062, Kampala Uganda Section Objectives Going through this section will enrich you with skill to be able to: diagnose nematode problems in the field considering all aspects involved in sampling, extraction and counting of nematodes from soil and plant parts, make permanent mounts, set up and maintain nematode cultures, design experimental set-ups for tests with nematodes Section Content sampling and quantification of nematodes extraction methods for plant-parasitic nematodes, free-living nematodes from soil and plant parts mounting of nematodes, drawing and measuring of nematodes, preparation of nematode inoculum and culturing nematodes, set-up of tests for research with plant-parasitic nematodes, A. Nematode sampling Unlike some pests and diseases, nematodes cannot be monitored by observation in the field. Nematodes must be extracted for microscopic examination in the laboratory. Nematodes can be collected by sampling soil and plant materials. There is no problem in finding nematodes, but getting the species and numbers you want may be trickier. In general, natural and undisturbed habitats will yield greater diversity and more slow-growing nematode species, while temporary and/or disturbed habitats will yield fewer and fast- multiplying species. Sampling considerations Getting nematodes in a sample that truly represent the underlying population at a given time requires due attention to sample size and depth, time and pattern of sampling, and handling and storage of samples.