193 Molecular, Morphological and Thermal Characters of 19 Pratylenchus Spp. and Relatives Using the D3 Segment of the Nuclear Ls
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathogenicity of Pratylenchus Hexincisus on Corn, Soybean, And
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1979 Pathogenicity of Pratylenchus hexincisus on corn, soybean, and tomato and population changes as influenced by hosts, temperature and soil type Mohammad Esmail Zirakparvar Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agricultural Science Commons, Agronomy and Crop Sciences Commons, and the Plant Pathology Commons Recommended Citation Zirakparvar, Mohammad Esmail, "Pathogenicity of Pratylenchus hexincisus on corn, soybean, and tomato and population changes as influenced by hosts, temperature and soil type" (1979). Retrospective Theses and Dissertations. 7260. https://lib.dr.iastate.edu/rtd/7260 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. -

Influence of Pratylenchus Vulnus and Meloidogyne Hapla on the Growth of Rootstocks of Rose~ G
influence of Pratylenchus vulnus and Meloidogyne hapla on the Growth of Rootstocks of Rose~ G. S. SANTO 2 and BERT LEAR '~ Abstract: Pratylenchus vulnus is involved in a disease of Rosa noisettiana 'Manetti" rose rootstock characterized by darkening of roots, death of feeder toots, and stunting of entire plants. The disease is more severe when plants are grown in silt loam soil than when they are grown in sandy loam soil. The nematodes reproduce best in silt loam soil at 20 C. Meloidogyne hapla did not affect the growth of Manetti. Rosa sp. 'Dr. Huey', Manetti, and R. odorata rose rootstocks were found to be good hosts for P. vulnus whereas R. multiflora was less suitable. M. hapla re- produced well on R. odorata, Dr. Huey, and R. multi[lora, but not on Maneni. Key Words: root- lesion nematode, root-knot nematode, reproduction, soil temperature, soil type. Pratylenchus vulnus Allen and Jensen roses was associated with a reduction in the was first reported from rose roots in Cali- population of M. hapla and Xiphinema fornia in 1953 (14). A 1970 survey of com- arnericanum Cobb as a result of multiple mercial rose greenhouses in northern Cali- applications of 1,2-dibromo-3-chloropro- fornia shows that this nematode is now pane (DBCP) (8). widely distributed (9), and Allen and Jen- Rosa noisettiana Thory 'Manetti' is the sen (l) also report that P. vulnus is widely most popular rootstock used in growing distributed on field-grown roses in southern greenhouse roses for cut flowers. R. odorata California. Sweet and Rosa sp. -

Nematodes and Agriculture in Continental Argentina
Fundam. appl. NemalOl., 1997.20 (6), 521-539 Forum article NEMATODES AND AGRICULTURE IN CONTINENTAL ARGENTINA. AN OVERVIEW Marcelo E. DOUCET and Marîa M.A. DE DOUCET Laboratorio de Nematologia, Centra de Zoologia Aplicada, Fant/tad de Cien.cias Exactas, Fisicas y Naturales, Universidad Nacional de Cordoba, Casilla df Correo 122, 5000 C6rdoba, Argentina. Acceplecl for publication 5 November 1996. Summary - In Argentina, soil nematodes constitute a diverse group of invertebrates. This widely distributed group incJudes more than twO hundred currently valid species, among which the plant-parasitic and entomopathogenic nematodes are the most remarkable. The former includes species that cause damages to certain crops (mainly MeloicU:igyne spp, Nacobbus aberrans, Ditylenchus dipsaci, Tylenchulus semipenetrans, and Xiphinema index), the latter inc1udes various species of the Mermithidae family, and also the genera Steinernema and Helerorhabditis. There are few full-time nematologists in the country, and they work on taxonomy, distribution, host-parasite relationships, control, and different aspects of the biology of the major species. Due tO the importance of these organisms and the scarcity of information existing in Argentina about them, nematology can be considered a promising field for basic and applied research. Résumé - Les nématodes et l'agriculture en Argentine. Un aperçu général - Les nématodes du sol représentent en Argentine un groupe très diversifiè. Ayant une vaste répartition géographique, il comprend actuellement plus de deux cents espèces, celles parasitant les plantes et les insectes étant considèrées comme les plus importantes. Les espèces du genre Me/oi dogyne, ainsi que Nacobbus aberrans, Dùylenchus dipsaci, Tylenchulus semipenetrans et Xiphinema index représentent un réel danger pour certaines cultures. -

Download Article (PDF)
OCCASIONAL PAPER NO. 240 Stlldies on plant and soil Nematodes associated with crops of economic importance in Gujarat QAISER H. BAQRI PADMA BOHRA ZOOLOGICAL SURVEY OF INDIA OCCASIONAL PAPER NO. 240 RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA Studies on plant and soil Nematodes associated with crops of economic importance in Gujarat. QAISER H. BAQRI AND PADMA BOHRA Desert Regional Station, Zoological Survey of India, Pali Road, Jhalan1and, Jodhpur - 342 005 Edited by the Director, Zoological Survey of India, Kolkata. Zoological Survey of India Kolkata CITATION Dr. BAQRI Qaiser H. & Dr. BOHRA Padma., 2005. Studies on Plant and Soil Nematodes Associated with Crops of Economic Importance in Gujarat of the Zoological Survey of India, : Rec. zool. Surv. India, Occasional Paper No. 240 : 1-48 (Published by the Director, Zoo I. Surv. India) Published : July, 2005 ISBN 81-8171-076-2 © Govt. of India, 2005 ALL RIGHTS RESERVED • No part of this publication may be reproduced stored in a retrieval system or transmitted in any form or by any means, el~ctronic, mechanical, photocopying, recording or otherwise without the prior permission of the publisher. • This book is sold subject to the condition that it shall not, by way of trade, be lent, resold hired out or otherwise disposed of without the publisher's consent, in an form of binding or cover other than that in which, it is published. • The correct price of this publication is the price printed on this page. Any revised price indicated by a rubber stamp or by a sticker or by any other means is incorrect and should be unacceptable. -

ENTO-364 (Introducto
K. K. COLLEGE OF AGRICULTURE, NASHIK DEPARTMENT OF AGRICULTURAL ENTOMOLOGY THEORY NOTES Course No.:- ENTO-364 Course Title: - Introductory Nematology Credits: - 2 (1+1) Compiled By Prof. T. B. Ugale & Prof. A. S. Mochi Assistant Professor Department of Agricultural Entomology 0 Complied by Prof. T. B. Ugale & Prof. A. S. Mochi (K. K. Wagh College of Agriculture, Nashik) TEACHING SCHEDULE Semester : VI Course No. : ENTO-364 Course Title : Introductory Nematology Credits : 2(1+1) Lecture Topics Rating No. 1 Introduction- History of phytonematology and economic 4 importance. 2 General characteristics of plant parasitic nematodes. 2 3 Nematode- General morphology and biology. 4 4 Classification of nematode up to family level with 4 emphasis on group of containing economical importance genera (Taxonomic). 5 Classification of nematode by habitat. 2 6 Identification of economically important plant nematodes 4 up to generic level with the help of key and description. 7 Symptoms caused by nematodes with examples. 4 8 Interaction of nematodes with microorganism 4 9 Different methods of nematode management. 4 10 Cultural methods 4 11 Physical methods 2 12 Biological methods 4 13 Chemical methods 2 14 Entomophilic nematodes- Species Biology 2 15 Mode of action 2 16 Mass production techniques for EPN 2 Reference Books: 1) A Text Book of Plant Nematology – K. D. Upadhay & Kusum Dwivedi, Aman Publishing House 2) Fundamentals of Plant Nematology – E. J. Jonathan, S. Kumar, K. Deviranjan, G. Rajendran, Devi Publications, 8, Couvery Nagar, Karumanolapam, Trichirappalli, 620 001. 3) Plant Nematodes - Methodology, Morphology, Systematics, Biology & Ecology Majeebur Rahman Khan, Department of Plant Protection, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh, India. -

Silencing Parasitism Effectors of the Root Lesion Nematode, Pratylenchus Thornei
Silencing parasitism effectors of the root lesion nematode, Pratylenchus thornei. This thesis is presented for the degree of Doctor of Philosophy of Murdoch University by Sameer Dilip Khot B.Sc. (Botany) & M.Sc. (Plant Pathology & Mycology), University of Mumbai, India M.S. (Plant Pathology), North Dakota State University, USA Western Australian State Agricultural Biotechnology Centre School of Veterinary and Life Sciences Murdoch University Perth, Western Australia 2018 1 DECLARATION I declare that this thesis is my own account of my research and contains as its main content work which has not previously been submitted for a degree at any tertiary education institution. Signature: Sameer D. Khot Date: 22-01-2018 2 ABSTRACT The root lesion nematode (RLN), Pratylenchus thornei, is a biotrophic migratory pest of plant roots and its infestation causes losses in many economically important crops. RNA interference (RNAi) is a naturally occurring eukaryotic phenomenon and can be used to silence parasitism effector genes of P. thornei using host-mediated RNAi. This may be developed as an environmentally friendly and a cost-effective control strategy. The overall aims of this research were to investigate the effects of in vitro and in planta RNAi silencing of putative P. thornei parasitism effector genes, and their nematicidal effects in two host plants. Five putative target parasitism genes vital for nematode entry into roots (Pt-Eng-1, Pt- PL), feeding (Pt-CLP) and suppressing host defence responses (Pt-UEP, Pt-GST) were identified, validated in silico using comparative bioinformatics, cloned into suitable in vitro transcription and binary vectors, and advanced to RNAi studies. -

Diversity, Phylogeny, Characterization and Diagnostics of Root-Knot and Lesion Nematodes
Diversity, phylogeny, characterization and diagnostics of root-knot and lesion nematodes Toon Janssen Promotors: Prof. Dr. Wim Bert Prof. Dr. Gerrit Karssen Thesis submitted to obtain the degree of doctor in Sciences, Biology Proefschrift voorgelegd tot het bekomen van de graad van doctor in de Wetenschappen, Biologie 1 Table of contents Acknowledgements Chapter 1: general introduction 1 Organisms under study: plant-parasitic nematodes .................................................... 11 1.1 Pratylenchus: root-lesion nematodes ..................................................................................... 13 1.2 Meloidogyne: root-knot nematodes ....................................................................................... 15 2 Economic importance ..................................................................................................... 17 3 Identification of plant-parasitic nematodes .................................................................. 19 4 Variability in reproduction strategies and genome evolution ..................................... 22 5 Aims .................................................................................................................................. 24 6 Outline of this study ........................................................................................................ 25 Chapter 2: Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. 1 Abstract -

Observations on the Genus Doronchus Andrássy
Vol. 20, No. 1, pp.91-98 International Journal of Nematology June, 2010 Occurrence and distribution of nematodes in Idaho crops Saad L. Hafez*, P. Sundararaj*, Zafar A. Handoo** and M. Rafiq Siddiqi*** *University of Idaho, 29603 U of I Lane, Parma, Idaho 83660, USA **USDA-ARS-Nematology Laboratory, Beltsville, Maryland 20705, USA ***Nematode Taxonomy Laboratory, 24 Brantwood Road, Luton, LU1 1JJ, England, UK E-mail: [email protected] Abstract. Surveys were conducted in Idaho, USA during the 2000-2006 cropping seasons to study the occurrence, population density, host association and distribution of plant-parasitic nematodes associated with major crops, grasses and weeds. Eighty-four species and 43 genera of plant-parasitic nematodes were recorded in soil samples from 29 crops in 20 counties in Idaho. Among them, 36 species are new records in this region. The highest number of species belonged to the genus Pratylenchus; P. neglectus was the predominant species among all species of the identified genera. Among the endoparasitic nematodes, the highest percentage of occurrence was Pratylenchus (29.7) followed by Meloidogyne (4.4) and Heterodera (3.4). Among the ectoparasitic nematodes, Helicotylenchus was predominant (8.3) followed by Mesocriconema (5.0) and Tylenchorhynchus (4.8). Keywords. Distribution, Helicotylenchus, Heterodera, Idaho, Meloidogyne, Mesocriconema, population density, potato, Pratylenchus, survey, Tylenchorhynchus, USA. INTRODUCTION and cropping systems in Idaho are highly conducive for nematode multiplication. Information concerning the revious reports have described the association of occurrence and distribution of nematodes in Idaho is plant-parasitic nematode species associated with important to assess their potential to cause economic damage P several crops in the Pacific Northwest (Golden et al., to many crop plants. -

A Reappraisal of Tylenchina (Nemata) 1
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1987 A Reappraisal of Tylenchina (Nemata) 1. For a New Approach to the Taxonomy of Tylenchina Michel Luc Muséun1 national d'Histoire naturelle Armand R. Maggenti University of California - Davis Renaud Fortuner California Department of Food and Agriculture Dewey J. Raski University of California - Davis Etienne Geraert Instituut voor Dierkunde Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Luc, Michel; Maggenti, Armand R.; Fortuner, Renaud; Raski, Dewey J.; and Geraert, Etienne, "A Reappraisal of Tylenchina (Nemata) 1. For a New Approach to the Taxonomy of Tylenchina" (1987). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 109. https://digitalcommons.unl.edu/parasitologyfacpubs/109 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Tribune A REAPPRAISAL OF "IYL,ENCHINA (NEMATA) 1. FOR A NEW APPROACH TO THE TAXONOMY OF TYLENCHINA Michel LUC",Armand R. MAGGENTI**, Renaud FORTUNER***, Dewey J. RASKI** and Etienne GERAERT**** * Muséun1 national d'Histoire naturelle, Laboratoire des Vers, 61, rue de Buffon, 75005 Paris; ** Division of Nematology, University of California, Davis, CA 95616, USA; *** California Department of Food and Agriculture, Analysis and Identification (Nematology), 1220 N. Street, Sacramento, CA 95814, USA, and **** Rijksuniversiteit Gent, Instituut voor Dierkunde, Ledeganckstraat 35, 9000 Gent, Belgium. -

Sudan University of Science and Technology College of Graduate
Sudan University of Science and Technology College of Graduate Studies Studies on Plant Parasitic Nematodes on Banana in Sennar and Kassala States دراﺳﺎت ﻋﻠﻰ اﻟﻨﯿﻤﺎﺗﻮدا اﻟﻤﺘﻄﻔﻠﺔ ﻋﻠﻰ ﻧﺒﺎت اﻟﻤﻮز ﻓﻰ وﻻﯾﺘﻰ ﺳﻨﺎر وﻛﺴﻼ A thesis submitted in to the Sudan University of Science and Technology in Fulfillment of the requirement for M.Sc. (Agric) in crop protection (Plant Pathology) By Duria Abdelsalam Eltahir Elshakh Supervisor: Dr. Ibrahim Saeed Mohammed 2014 ﺑﺳم ﷲ اﻟرﺣﻣن اﻟرﺣﯾم Sudan University of Science and Technology College of Graduate Studies Studies on Plant Parasitic Nematodes on Banana in Sennar and Kassala States دراﺳﺎت ﻋﻠﻰ اﻟﻨﯿﻤﺎﺗﻮدا اﻟﻤﺘﻄﻔﻠﺔ ﻋﻠﻰ ﻧﺒﺎت اﻟﻤﻮز ﻓﻰ وﻻﯾﺘﻰ ﺳﻨﺎر وﻛﺴﻼ A thesis submitted in the requirement of the Degree of M.Sc. Agric. In Crop Protection (Plant Pathology) By Duria Abdelsalam Eltahir Elshakh B.Sc. in Crop Protection University of Zagazig, Egypt(1986) Main Supervisor: Dr. Ibrahim Saeed Mohammed Co. Supervisor: Prof. Gamal Abdalla Elbadri 2014 Dedication To the soul of my father Lovely mother Intimate husband Brothers and sisters To all those who search of knowledge 3 ACKNOWLEDGMENT Thanks and praise to the Beneficent and Merciful God who provided me with health, strength and patience to have this drop from the renewable flood of knowledge. I am gratefully to my supervisors, Doctor Ibrahim Saeed Mohammed and Professor Gamal Abdalla Elbadri for all the encouragement, helpful guidance and continued support. Special thankS to my Co. supervisor Professor. Gamal Abdalla Elbadri under who's the supervision of work has been successfully carried out with his invaluable advices, unlimited helpful and for this correction of this study. -
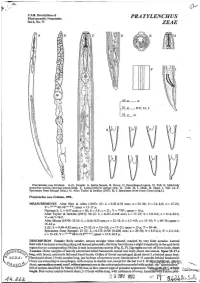
Pratylenchus Zeae
/p C.I.H. Descriptions of Plant-parasitic Nematodes PRATYLENCHUS Set 6, No. 77 ZEAE ' A D F V L 50 P A i5 ,B-F,H,I 25P ,G G Prutylenchus zeue Graham. A-G. Female. A. Entire female. B. Ovary. C. Oesophageal region. D. Tail. E. Mid-body ' transverse section showing lateral fields. F. Lateral field in surface view. G. Tails. H, I. Male. H. Head. I. Tail. (A-F. Specimens from Senegal (orig.). G. After Taylor & Jenkins (1957). H, I. Specimens from Ivory Coast (orig.).) PratyZemhus zeae Graham, 1951. MEASUREMENTS After Sher & Allen (1953): 99: L = 0.36-0.58 mm; a = 25-30; b = 5.4-8.0; c= 17-21; V = 26-43 68-763.4-6."; spear = 15-17 p. Neotype 9: L = 0.47 mm; a = 26; b = 5.9; c = 21 ; V = 30704;spear = 16 p. After Taylor & Jenkins (1957): 90 99: L = 0.413-0.639 mm; a = 17-25; b = 5.0-9.6; c = 11.2-24.1; v = 64.7-74.9. After Memy (1970): 25 99: L = 0.34-0.55 mm; a = 22-33; b = 3.3-4.9; c = 13-18; V = 69-74; spear = 15-18 p. 5 88: L = 0.40-0.42 mm; a = 27-32; b = 3.6-5.0; c = 17-21 ;spear = 15 p; T = 30-44. Specimens from Senegal: 25 99: L = 0.373-0.506 (0.428) mm; a = 20-30; b = 4.9-6.1 ; b' = 3.2-4.6; c = 15-19; V = 23-38 68.6-73.93-86.7; spear= 15.5-16.5 p. -

Nematodes As Bioindicators of Soil Food Web
NEMATODES AS BIOINDICATORS OF SOIL FOOD WEB HEALTH IN AGROECOSYSTEMS: A CRITICAL ANALYSIS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By SHABEG SINGH BRIAR * * * * * The Ohio State University 2007 Dissertation Committee: Professor Parwinder S. Grewal, Adviser Professor Sally A. Miller, Adviser Professor Casey W. Hoy Professor Landon H. Rhodes Approved by Advisers ____________________ ____________________ Plant Pathology Graduate Program Abstract Nematodes occupy a central position in the soil food web occurring at multiple trophic levels and, therefore, have the potential to provide insights into condition of the soil food webs. I hypothesized that differences in management strategies may have differential effects on nematode community structure and soil properties. This hypothesis was tested in three different replicated experiments. In the first study a conventional farming system receiving synthetic inputs was compared with an organically managed system and in the second study four different farming strategies with and without compost application transitioning to organic management were compared for nematode communities and soil characteristics including soil bulk density, organic matter, microbial biomass and mineral-N. The third study was aimed at assessing the indicative value of various nematode measures in five habitats. Nematode food webs were analyzed for trophic group abundance and by calculating MI, and enrichment (EI), structure (SI) and channel indices (CI) based on weighted abundance of c-p (colonizer-persister) guilds. Bacterivore nematodes were more abundant in the organic than the conventional whereas the conventional system had higher population of the root lesion nematode, Pratylenchus crenatus compared with organic system.