Sudan University of Science and Technology College of Graduate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influence of Pratylenchus Vulnus and Meloidogyne Hapla on the Growth of Rootstocks of Rose~ G
influence of Pratylenchus vulnus and Meloidogyne hapla on the Growth of Rootstocks of Rose~ G. S. SANTO 2 and BERT LEAR '~ Abstract: Pratylenchus vulnus is involved in a disease of Rosa noisettiana 'Manetti" rose rootstock characterized by darkening of roots, death of feeder toots, and stunting of entire plants. The disease is more severe when plants are grown in silt loam soil than when they are grown in sandy loam soil. The nematodes reproduce best in silt loam soil at 20 C. Meloidogyne hapla did not affect the growth of Manetti. Rosa sp. 'Dr. Huey', Manetti, and R. odorata rose rootstocks were found to be good hosts for P. vulnus whereas R. multiflora was less suitable. M. hapla re- produced well on R. odorata, Dr. Huey, and R. multi[lora, but not on Maneni. Key Words: root- lesion nematode, root-knot nematode, reproduction, soil temperature, soil type. Pratylenchus vulnus Allen and Jensen roses was associated with a reduction in the was first reported from rose roots in Cali- population of M. hapla and Xiphinema fornia in 1953 (14). A 1970 survey of com- arnericanum Cobb as a result of multiple mercial rose greenhouses in northern Cali- applications of 1,2-dibromo-3-chloropro- fornia shows that this nematode is now pane (DBCP) (8). widely distributed (9), and Allen and Jen- Rosa noisettiana Thory 'Manetti' is the sen (l) also report that P. vulnus is widely most popular rootstock used in growing distributed on field-grown roses in southern greenhouse roses for cut flowers. R. odorata California. Sweet and Rosa sp. -

Endophytic Control of Cosmopolites Sordidus and Radopholus Similis Using Fusarium Oxysporum V5w2 in Tissue Culture Banana
Endophytic control of Cosmopolites sordidus and Radopholus similis using Fusarium oxysporum V5w2 in tissue culture banana Dennis M.W. Ochieno Thesis committee Thesis supervisors Prof. dr. Marcel Dicke Professor of Entomology Wageningen University Prof. dr. ir. Arnold van Huis Personal Chair at the Laboratory of Entomology Wageningen University Thesis co-supervisor Dr. Thomas Dubois Biocontrol Specialist International Institute of Tropical Agriculture Other members Prof. dr. TWM Kuijper, Wageningen University Prof. dr. RA Sikora, University of Bonn, Germany Dr. JM Raaijmakers, Wageningen University Dr. MNEJ Smit, IPM specialist, (private consultant) This research was conducted under the auspices of the C. T. de Wit Graduate School of Production Ecology and Resource Conservation Endophytic control of Cosmopolites sordidus and Radopholus similis using Fusarium oxysporum V5w2 in tissue culture banana Dennis M.W. Ochieno Thesis submitted in partial fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. dr. M.J. Kropff in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Monday 1 November 2010 at 11 a.m. in the Aula Dennis M.W. Ochieno Endophytic control of Cosmopolites sordidus and Radopholus similis using Fusarium oxysporum V5w2 in tissue culture banana Thesis, Wageningen University, Wageningen, NL (2010) With references, with summaries in Dutch and English ISBN 978-90-8585-637-5 Table of contents Acknowledgements vii Abstract -

ENTO-364 (Introducto
K. K. COLLEGE OF AGRICULTURE, NASHIK DEPARTMENT OF AGRICULTURAL ENTOMOLOGY THEORY NOTES Course No.:- ENTO-364 Course Title: - Introductory Nematology Credits: - 2 (1+1) Compiled By Prof. T. B. Ugale & Prof. A. S. Mochi Assistant Professor Department of Agricultural Entomology 0 Complied by Prof. T. B. Ugale & Prof. A. S. Mochi (K. K. Wagh College of Agriculture, Nashik) TEACHING SCHEDULE Semester : VI Course No. : ENTO-364 Course Title : Introductory Nematology Credits : 2(1+1) Lecture Topics Rating No. 1 Introduction- History of phytonematology and economic 4 importance. 2 General characteristics of plant parasitic nematodes. 2 3 Nematode- General morphology and biology. 4 4 Classification of nematode up to family level with 4 emphasis on group of containing economical importance genera (Taxonomic). 5 Classification of nematode by habitat. 2 6 Identification of economically important plant nematodes 4 up to generic level with the help of key and description. 7 Symptoms caused by nematodes with examples. 4 8 Interaction of nematodes with microorganism 4 9 Different methods of nematode management. 4 10 Cultural methods 4 11 Physical methods 2 12 Biological methods 4 13 Chemical methods 2 14 Entomophilic nematodes- Species Biology 2 15 Mode of action 2 16 Mass production techniques for EPN 2 Reference Books: 1) A Text Book of Plant Nematology – K. D. Upadhay & Kusum Dwivedi, Aman Publishing House 2) Fundamentals of Plant Nematology – E. J. Jonathan, S. Kumar, K. Deviranjan, G. Rajendran, Devi Publications, 8, Couvery Nagar, Karumanolapam, Trichirappalli, 620 001. 3) Plant Nematodes - Methodology, Morphology, Systematics, Biology & Ecology Majeebur Rahman Khan, Department of Plant Protection, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh, India. -

Silencing Parasitism Effectors of the Root Lesion Nematode, Pratylenchus Thornei
Silencing parasitism effectors of the root lesion nematode, Pratylenchus thornei. This thesis is presented for the degree of Doctor of Philosophy of Murdoch University by Sameer Dilip Khot B.Sc. (Botany) & M.Sc. (Plant Pathology & Mycology), University of Mumbai, India M.S. (Plant Pathology), North Dakota State University, USA Western Australian State Agricultural Biotechnology Centre School of Veterinary and Life Sciences Murdoch University Perth, Western Australia 2018 1 DECLARATION I declare that this thesis is my own account of my research and contains as its main content work which has not previously been submitted for a degree at any tertiary education institution. Signature: Sameer D. Khot Date: 22-01-2018 2 ABSTRACT The root lesion nematode (RLN), Pratylenchus thornei, is a biotrophic migratory pest of plant roots and its infestation causes losses in many economically important crops. RNA interference (RNAi) is a naturally occurring eukaryotic phenomenon and can be used to silence parasitism effector genes of P. thornei using host-mediated RNAi. This may be developed as an environmentally friendly and a cost-effective control strategy. The overall aims of this research were to investigate the effects of in vitro and in planta RNAi silencing of putative P. thornei parasitism effector genes, and their nematicidal effects in two host plants. Five putative target parasitism genes vital for nematode entry into roots (Pt-Eng-1, Pt- PL), feeding (Pt-CLP) and suppressing host defence responses (Pt-UEP, Pt-GST) were identified, validated in silico using comparative bioinformatics, cloned into suitable in vitro transcription and binary vectors, and advanced to RNAi studies. -

Diversity, Phylogeny, Characterization and Diagnostics of Root-Knot and Lesion Nematodes
Diversity, phylogeny, characterization and diagnostics of root-knot and lesion nematodes Toon Janssen Promotors: Prof. Dr. Wim Bert Prof. Dr. Gerrit Karssen Thesis submitted to obtain the degree of doctor in Sciences, Biology Proefschrift voorgelegd tot het bekomen van de graad van doctor in de Wetenschappen, Biologie 1 Table of contents Acknowledgements Chapter 1: general introduction 1 Organisms under study: plant-parasitic nematodes .................................................... 11 1.1 Pratylenchus: root-lesion nematodes ..................................................................................... 13 1.2 Meloidogyne: root-knot nematodes ....................................................................................... 15 2 Economic importance ..................................................................................................... 17 3 Identification of plant-parasitic nematodes .................................................................. 19 4 Variability in reproduction strategies and genome evolution ..................................... 22 5 Aims .................................................................................................................................. 24 6 Outline of this study ........................................................................................................ 25 Chapter 2: Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. 1 Abstract -

A Reappraisal of Tylenchina (Nemata) 1
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1987 A Reappraisal of Tylenchina (Nemata) 1. For a New Approach to the Taxonomy of Tylenchina Michel Luc Muséun1 national d'Histoire naturelle Armand R. Maggenti University of California - Davis Renaud Fortuner California Department of Food and Agriculture Dewey J. Raski University of California - Davis Etienne Geraert Instituut voor Dierkunde Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Luc, Michel; Maggenti, Armand R.; Fortuner, Renaud; Raski, Dewey J.; and Geraert, Etienne, "A Reappraisal of Tylenchina (Nemata) 1. For a New Approach to the Taxonomy of Tylenchina" (1987). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 109. https://digitalcommons.unl.edu/parasitologyfacpubs/109 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Tribune A REAPPRAISAL OF "IYL,ENCHINA (NEMATA) 1. FOR A NEW APPROACH TO THE TAXONOMY OF TYLENCHINA Michel LUC",Armand R. MAGGENTI**, Renaud FORTUNER***, Dewey J. RASKI** and Etienne GERAERT**** * Muséun1 national d'Histoire naturelle, Laboratoire des Vers, 61, rue de Buffon, 75005 Paris; ** Division of Nematology, University of California, Davis, CA 95616, USA; *** California Department of Food and Agriculture, Analysis and Identification (Nematology), 1220 N. Street, Sacramento, CA 95814, USA, and **** Rijksuniversiteit Gent, Instituut voor Dierkunde, Ledeganckstraat 35, 9000 Gent, Belgium. -

Towards Management of Musa Nematodes in Asia and the Pacific
The mission of the International Network for the Improvement of Banana and Plantain (INIBAP) is to sustainably increase the productivity of banana and plantain grown on smallholdings for domestic consumption and for local and export markets. The programme has four specific objectives: To organize and coordinate a global research effort on banana and plantain, aimed at the development, evaluation and dissemination of improved banana cultivars and at the conservation and use of Musa diversity. To promote and strengthen collaboration and partnerships in banana-related activities at the national, regional and global levels. To strengthen the ability of NARS to conduct research and development activities on bananas and plantains. To coordinate, facilitate and support the production, collection and exchange of information and documentation related to banana and plantain. INIBAP is a network of the International Plant Genetic Resources Institute (IPGRI), a Future Harvest center. The International Plant Genetic Resources Institute (IPGRI) is an independent international scientific organization that seeks to advance the conservation and use of plant genetic diversity for the well-being of present and future generations. It is one of the 16 Future Harvest Centres supported by the Consultative Group on International Agricultural Research (CGIAR), an association of public and private members who support efforts to mobilize cutting-edge science to reduce hunger and proverty, improve human nutrition and health, and protect the environment. IPGRI has its headquarters in Maccarese, near Rome, Italy, with offices in more than 20 other countries worldwide. The Institute operates through three programmes: (1) the Plant Genetic Resources Programme, (2) the CGIAR Genetic Resources Support Programme and (3) the International Network for the Improvement of Banana and Plantain (INIBAP). -

Radopholus Similis
Feb 20Pathogen of the month – Feb 2020 F M F sp v M a b c d e f g Fig. 1. (a) Mature and juvenile nematodes expressing auto-fluorescent globules/lip region and egg with no autofluorescence; (b) and (c) female (F) and male (M) nematodes highlighting the differences in morphology in the lip region (black arrows) and reproductive organs i.e. (sp) spicules and (v) vulva; (d) female nematode undergoing molting; (e) Nematophagous fungal growth within a dead burrowing nematode on 1% (w/v) water agar plate; (f) root lesions in R. similis infected banana roots and (g) banana plant toppling due to burrowing nematode damage. Scale bars represent 100 mm, 20 mm, 50 mm and 200 mm in (a), (b), (c) and (e) respectively. Common Name: Burrowing nematode Disease: Spreading decline (citrus); toppling or blackhead disease (banana); yellows disease (black pepper) Classification: K: Animalia P: Nematoda C: Secementea O: Tylenchida F: Pratylenchidae First observed in Fiji in 1893, the burrowing nematode, Radopholus similis infects the roots of many commercial crops such as banana, ginger and citrus. A draft genome is available and sequence analysis suggests that it is closely related to cyst nematodes than to root knot or other migratory endoparasitic nematodes such as Pratylenchus. Recent work on R. similis isolates from Fiji and Australia differed in pathogenicity on ginger and banana, indicating pathotype differences between isolates. Biology and Ecology: All larval stages and adult females Distribution: It is found in most tropical and subtropical of Radopholus similis are infective and feed on root cortical regions of the world. -

Functional Diversity of Soil Nematodes in Relation to the Impact of Agriculture—A Review
diversity Review Functional Diversity of Soil Nematodes in Relation to the Impact of Agriculture—A Review Stela Lazarova 1,* , Danny Coyne 2 , Mayra G. Rodríguez 3 , Belkis Peteira 3 and Aurelio Ciancio 4,* 1 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Y. Gagarin Str., 1113 Sofia, Bulgaria 2 International Institute of Tropical Agriculture (IITA), Kasarani, Nairobi 30772-00100, Kenya; [email protected] 3 National Center for Plant and Animal Health (CENSA), P.O. Box 10, Mayabeque Province, San José de las Lajas 32700, Cuba; [email protected] (M.G.R.); [email protected] (B.P.) 4 Consiglio Nazionale delle Ricerche, Istituto per la Protezione Sostenibile delle Piante, 70126 Bari, Italy * Correspondence: [email protected] (S.L.); [email protected] (A.C.); Tel.: +359-8865-32-609 (S.L.); +39-080-5929-221 (A.C.) Abstract: The analysis of the functional diversity of soil nematodes requires detailed knowledge on theoretical aspects of the biodiversity–ecosystem functioning relationship in natural and managed terrestrial ecosystems. Basic approaches applied are reviewed, focusing on the impact and value of soil nematode diversity in crop production and on the most consistent external drivers affecting their stability. The role of nematode trophic guilds in two intensively cultivated crops are examined in more detail, as representative of agriculture from tropical/subtropical (banana) and temperate (apple) climates. The multiple facets of nematode network analysis, for management of multitrophic interactions and restoration purposes, represent complex tasks that require the integration of different interdisciplinary expertise. Understanding the evolutionary basis of nematode diversity at the field Citation: Lazarova, S.; Coyne, D.; level, and its response to current changes, will help to explain the observed community shifts. -

Past and Present of the Nematode Radopholus Similis (Cobb)
CROP PROTECTION Past and present of the nematode Radopholus similis (Cobb) Thorne with emphasis on Musa: a review Pasado y presente del nematodo Radopholus similis (Cobb) Thorne con énfasis en musáceas: una revisión Charles Volcy1 ABSTRACT RESUMEN This review presents a historical background on different as- En la presente reseña, se hace un recorrido histórico sobre pects of the nematode Radopholus similis (Cobb) Thorne, an diferentes aspectos del nematodo Radopholus similis (Cobb) important pathogen of Musa, black pepper and anthurium. Thorne, importante patógeno de musáceas, de la pimienta It examines the history of its discovery and its name, new negra y del anturio. Se analiza la historia de su descubrimiento approaches to its taxonomy and its genome, and the process y de su nombre, los nuevos planteamientos sobre su taxonomía of dissemination and pathogenic variability. Finally, it docu- y su genoma, su proceso de diseminación y su variabilidad ments the evolution of the control measures in banana, from patogénica. Finalmente, se documenta la evolución de las me- chemical control to bio-protection and managing the habitat didas de control en banano, desde el control químico hasta la of the pathogen. bioprotección y el manejo del hábitat del patógeno. Key words: history, taxonomy, pathogenicity, management. Palabras clave: historia, taxonomía, patogenicidad, manejo. Introduction Origin and spread of R. similis Radopholus similis (Cobb), with the name “nematodo The discovery, the first morphometric description, and the barrenador” in Spanish and burrowing nematode or “Fiji first names of this nematode were the fruits of research by banana-root nematode” in English, is one of the ten most the American Nathan Cobb August in Australia after the important phyto-helminths in the tropics (Haegeman et pathological examination of banana roots collected in Fiji al., 2010). -
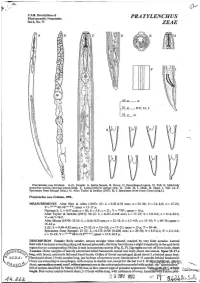
Pratylenchus Zeae
/p C.I.H. Descriptions of Plant-parasitic Nematodes PRATYLENCHUS Set 6, No. 77 ZEAE ' A D F V L 50 P A i5 ,B-F,H,I 25P ,G G Prutylenchus zeue Graham. A-G. Female. A. Entire female. B. Ovary. C. Oesophageal region. D. Tail. E. Mid-body ' transverse section showing lateral fields. F. Lateral field in surface view. G. Tails. H, I. Male. H. Head. I. Tail. (A-F. Specimens from Senegal (orig.). G. After Taylor & Jenkins (1957). H, I. Specimens from Ivory Coast (orig.).) PratyZemhus zeae Graham, 1951. MEASUREMENTS After Sher & Allen (1953): 99: L = 0.36-0.58 mm; a = 25-30; b = 5.4-8.0; c= 17-21; V = 26-43 68-763.4-6."; spear = 15-17 p. Neotype 9: L = 0.47 mm; a = 26; b = 5.9; c = 21 ; V = 30704;spear = 16 p. After Taylor & Jenkins (1957): 90 99: L = 0.413-0.639 mm; a = 17-25; b = 5.0-9.6; c = 11.2-24.1; v = 64.7-74.9. After Memy (1970): 25 99: L = 0.34-0.55 mm; a = 22-33; b = 3.3-4.9; c = 13-18; V = 69-74; spear = 15-18 p. 5 88: L = 0.40-0.42 mm; a = 27-32; b = 3.6-5.0; c = 17-21 ;spear = 15 p; T = 30-44. Specimens from Senegal: 25 99: L = 0.373-0.506 (0.428) mm; a = 20-30; b = 4.9-6.1 ; b' = 3.2-4.6; c = 15-19; V = 23-38 68.6-73.93-86.7; spear= 15.5-16.5 p. -

Integrated Management of Banana Nematodes: Lessons from a Case Study in the French West Indies
From Science to Field Banana Case Study – Guide Number 4 Integrated management of banana nematodes: Lessons from a case study in the French West Indies Jean-Michel Risède, CIRAD, France Tino daMbas, ITBAN, France Christian ChabRieR, CIRAD, France Raphaël aChaRd, CIRAD, France Marc doRel, CIRAD, France Patrick QuénéheRvé, IRD, France Association of bananas with the perennial legume Neonotonia wightii. © Jean-Michel Risède, CIRAD, France. Food Quality and Safety From Science to Field Banana Case Study – Guide Number 4 integrated management of banana nematodes: lessons from a case study in the French West indies Plant-parasitic nematodes are tiny worms that live mainly in soil and roots. In the case of banana plants, the most damaging species spend most of their life cycle in root and corm tissues. Their mouth cavity contains a hollow stylet with which they puncture the cell and remove the contents. Plurispecific communities of millions of individuals can develop in corm and root tissues of which they alter the physical and functional integrity. Nematode proliferation can disrupt nutrient and water uptake, delay growth and cause banana plants to topple over. In the French West Indies, toppling over is the main damage caused by nematodes. As in many other banana-producing regions around the world, 10 years ago in the French West Indies methods for the control of nematodes in export bananas relied on the use of synthetic carbamate and organophosphate nematicides. For the most part classified as toxic or highly toxic, in recent years many of these products have gradually been banned. Alter- native integrated plant-parasitic nematode management has consequently been developed in banana cropping systems in the French West Indies with the support of different stake- holders (growers, researchers, extension officers etc).