FINAL THESIS Submission Newest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathogenicity of Pratylenchus Hexincisus on Corn, Soybean, And
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1979 Pathogenicity of Pratylenchus hexincisus on corn, soybean, and tomato and population changes as influenced by hosts, temperature and soil type Mohammad Esmail Zirakparvar Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agricultural Science Commons, Agronomy and Crop Sciences Commons, and the Plant Pathology Commons Recommended Citation Zirakparvar, Mohammad Esmail, "Pathogenicity of Pratylenchus hexincisus on corn, soybean, and tomato and population changes as influenced by hosts, temperature and soil type" (1979). Retrospective Theses and Dissertations. 7260. https://lib.dr.iastate.edu/rtd/7260 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. -

Research/Investigación Aggressiveness Of
RESEARCH/INVESTIGACIÓN AGGRESSIVENESS OF PRATYLENCHUS BRACHYURUS TO SUGARCANE, COMPARED WITH KEY NEMATODE P. ZEAE Bruno Flávio Figueiredo Barbosa1*, Jaime Maia dos Santos¹, José Carlos Barbosa¹, Pedro Luiz Martins Soares1, Anderson Robert Ruas², Rafael Bernal de Carvalho¹ ¹Jaboticabal Unit, UNESP São Paulo State University, Department of Plant Protection, Jaboticabal, SP, 14884-900, Brazil. ²São Luiz College, Jaboticabal, SP, 14870-370, Brazil. The work is part of the doctorate thesis in Agronomy (Crop Production) of the first author. Author for correspondence: [email protected] ABSTRACT Barbosa, B. F. F., J. M. dos Santos, J. C. Barbosa, P. L. M. Soares, A. R. Ruas, R. B. de Carvalho. 2013. Aggressiveness of Pratylenchus brachyurus to the sugarcane, compared with key nematode P. zeae. Nematropica 43:119-130. Pratylenchus zeae, Meloidogyne javanica and M. incognita are considered key species of nematodes in sugarcane in Brazil, but P. brachyurus is also frequently found. This study was conducted to determine the aggressiveness of P. brachyurus compared with P. zeae to sugarcane. Plants were grown in pots (100 L) in an open area with initial inoculation of 10, 100, 1,000, 10,000 and 100,000/plant for P. brachyurus and P. zeae. The nematode inocula were from in vitro, carrot-cylinder cultures. Sampling was performed every 60 days until 300 days after inoculation. At harvest, we evaluated the population dynamics of the nematodes and plant growth characteristics. The population for the initial levels of 10 and 100,000 specimens/plant, for P. brachyurus and P. zeae at 300 days after inoculation were similar. This fact shows that, upon detection of nematodes in a certain place during the planting of sugarcane, the ratoon on this area should be treated so as to control populations of P. -

Nematodes and Agriculture in Continental Argentina
Fundam. appl. NemalOl., 1997.20 (6), 521-539 Forum article NEMATODES AND AGRICULTURE IN CONTINENTAL ARGENTINA. AN OVERVIEW Marcelo E. DOUCET and Marîa M.A. DE DOUCET Laboratorio de Nematologia, Centra de Zoologia Aplicada, Fant/tad de Cien.cias Exactas, Fisicas y Naturales, Universidad Nacional de Cordoba, Casilla df Correo 122, 5000 C6rdoba, Argentina. Acceplecl for publication 5 November 1996. Summary - In Argentina, soil nematodes constitute a diverse group of invertebrates. This widely distributed group incJudes more than twO hundred currently valid species, among which the plant-parasitic and entomopathogenic nematodes are the most remarkable. The former includes species that cause damages to certain crops (mainly MeloicU:igyne spp, Nacobbus aberrans, Ditylenchus dipsaci, Tylenchulus semipenetrans, and Xiphinema index), the latter inc1udes various species of the Mermithidae family, and also the genera Steinernema and Helerorhabditis. There are few full-time nematologists in the country, and they work on taxonomy, distribution, host-parasite relationships, control, and different aspects of the biology of the major species. Due tO the importance of these organisms and the scarcity of information existing in Argentina about them, nematology can be considered a promising field for basic and applied research. Résumé - Les nématodes et l'agriculture en Argentine. Un aperçu général - Les nématodes du sol représentent en Argentine un groupe très diversifiè. Ayant une vaste répartition géographique, il comprend actuellement plus de deux cents espèces, celles parasitant les plantes et les insectes étant considèrées comme les plus importantes. Les espèces du genre Me/oi dogyne, ainsi que Nacobbus aberrans, Dùylenchus dipsaci, Tylenchulus semipenetrans et Xiphinema index représentent un réel danger pour certaines cultures. -

Theory Manual Course No. Pl. Path
NAVSARI AGRICULTURAL UNIVERSITY Theory Manual INTRODUCTORY PLANT NEMATOLOGY Course No. Pl. Path 2.2 (V Dean’s) nd 2 Semester B.Sc. (Hons.) Agri. PROF.R.R.PATEL, ASSISTANT PROFESSOR Dr.D.M.PATHAK, ASSOCIATE PROFESSOR Dr.R.R.WAGHUNDE, ASSISTANT PROFESSOR DEPARTMENT OF PLANT PATHOLOGY COLLEGE OF AGRICULTURE NAVSARI AGRICULTURAL UNIVERSITY BHARUCH 392012 1 GENERAL INTRODUCTION What are the nematodes? Nematodes are belongs to animal kingdom, they are triploblastic, unsegmented, bilateral symmetrical, pseudocoelomateandhaving well developed reproductive, nervous, excretoryand digestive system where as the circulatory and respiratory systems are absent but govern by the pseudocoelomic fluid. Plant Nematology: Nematology is a science deals with the study of morphology, taxonomy, classification, biology, symptomatology and management of {plant pathogenic} nematode (PPN). The word nematode is made up of two Greek words, Nema means thread like and eidos means form. The words Nematodes is derived from Greek words ‘Nema+oides’ meaning „Thread + form‟(thread like organism ) therefore, they also called threadworms. They are also known as roundworms because nematode body tubular is shape. The movement (serpentine) of nematodes like eel (marine fish), so also called them eelworm in U.K. and Nema in U.S.A. Roundworms by Zoologist Nematodes are a diverse group of organisms, which are found in many different environments. Approximately 50% of known nematode species are marine, 25% are free-living species found in soil or freshwater, 15% are parasites of animals, and 10% of known nematode species are parasites of plants (see figure at left). The study of nematodes has traditionally been viewed as three separate disciplines: (1) Helminthology dealing with the study of nematodes and other worms parasitic in vertebrates (mainly those of importance to human and veterinary medicine). -

The Effect of Pratylenchus Zeae on the Growth and Yield of Upland Rice
The effect of Pratylenchus zeae on the growth and yield of upland rice Richard A. PLOWRIGHT",Danilo MATUS**, Tin AUNG**and Twng-Wah MEW** * CAB International Institute of Parasitology, 395 a, Hatfield Road, St. Albans, Hertfordshire, AL4 OXU, UK and ** International Rice Research Institute, P. O. Box 933, Manila, Philippines. SUMMARY The root lesion nematode Pratylenchus zeae is widely distributed on upland rice but its economic importance has not been assessed. In a field trial, following a five month clean fallow, the ofcontrol P. zeae using carbofuran, increased the yieldof cv. Upl Ri-5 whilst the yield of cv. Kinandang Patong was unaffected. Pre-sowing soil population densities (Pi) of P. zeae were low (0-1 11 nematodes/lOOml soil) and there were no obvious symptoms of infectionduring early vegetativegrowth although the plant height of Upl Ri-5 was slightly reduced. At harvest the yield of treated plants was increased byO/O 13-29of that of untreated plants having a mean infection of 1 350 nematodedg root(P < 0.05). In the glasshouse the rate of growth and tilleringof cv. IR36 was significantly reduced with a highPi (630-3 O00 nematodesllO0 cm3 soil). Infected root systems were stunted and mean root fresh weight was reduced by 40-60%. Although infection reducedthe no. of spikeletslplant, these plants had a higher harvest index and consequently grain yield was unaffected. The relationship between yield and the population density of P. zeae at different crop growth stages, in the field indicates low tolerance and a high relative minimum yield of 65O/o. RESUMÉ Influence de Pratylenchus zeae sur la croissance et la récolte du riz de plateau Pratylenchus zeae est très répandusur le riz de plateau mais son importance n'a jamais été évaluée. -

Observations on the Genus Doronchus Andrássy
Vol. 20, No. 1, pp.91-98 International Journal of Nematology June, 2010 Occurrence and distribution of nematodes in Idaho crops Saad L. Hafez*, P. Sundararaj*, Zafar A. Handoo** and M. Rafiq Siddiqi*** *University of Idaho, 29603 U of I Lane, Parma, Idaho 83660, USA **USDA-ARS-Nematology Laboratory, Beltsville, Maryland 20705, USA ***Nematode Taxonomy Laboratory, 24 Brantwood Road, Luton, LU1 1JJ, England, UK E-mail: [email protected] Abstract. Surveys were conducted in Idaho, USA during the 2000-2006 cropping seasons to study the occurrence, population density, host association and distribution of plant-parasitic nematodes associated with major crops, grasses and weeds. Eighty-four species and 43 genera of plant-parasitic nematodes were recorded in soil samples from 29 crops in 20 counties in Idaho. Among them, 36 species are new records in this region. The highest number of species belonged to the genus Pratylenchus; P. neglectus was the predominant species among all species of the identified genera. Among the endoparasitic nematodes, the highest percentage of occurrence was Pratylenchus (29.7) followed by Meloidogyne (4.4) and Heterodera (3.4). Among the ectoparasitic nematodes, Helicotylenchus was predominant (8.3) followed by Mesocriconema (5.0) and Tylenchorhynchus (4.8). Keywords. Distribution, Helicotylenchus, Heterodera, Idaho, Meloidogyne, Mesocriconema, population density, potato, Pratylenchus, survey, Tylenchorhynchus, USA. INTRODUCTION and cropping systems in Idaho are highly conducive for nematode multiplication. Information concerning the revious reports have described the association of occurrence and distribution of nematodes in Idaho is plant-parasitic nematode species associated with important to assess their potential to cause economic damage P several crops in the Pacific Northwest (Golden et al., to many crop plants. -

Grain Yield and Heterosis of Maize Hybrids Under Nematode Infested and Nematicide Treated Conditions
Journal of Nematology 43(3–4):209–219. 2011. Ó The Society of Nematologists 2011. Grain Yield and Heterosis of Maize Hybrids under Nematode Infested and Nematicide Treated Conditions 1,2 1 1 2 3 FRANK KAGODA, JOHN DERERA, PANGIRAYI TONGOONA, DANIEL L. COYNE, HERBERT L. TALWANA Abstract: Plant-parasitic nematodes are present on maize but resistant genotypes have not been identified in Uganda. This study was aimed at determining the level of nematode resistance among F1 hybrids, and to estimate grain yield, heterosis and yield losses associated with maize hybrids under nematode infestation. The 30 F1 hybrids and two local checks were evaluated in a split plot design with nematode treatment (nematode infested versus nematicide treated) as the whole plot factor, and the hybrids as subplot factors arranged in an 8 x 4 alpha-lattice design. The experiment was conducted simultaneously at three sites. The hybrids were also evaluated in a split plot design under greenhouse conditions at IITA-Namulonge. Results revealed 24 P. zeae susceptible hybrids compared to only six P. zeae resistant hybrids. Grain yield across sites was higher by about 400 kg ha-1 under nematicide treatment than under nematode infestation. The nematode tolerant/resistant hybrids exhibited yields ranging from 5.0 to 8.4 t ha-1 compared to 5.0 t ha-1 obtained from the best check. Grain yield loss was up to 28% among susceptible hybrids, indicating substantial economic yield losses due to nematodes. Under field conditions, desired heterosis was recorded on 18 hybrids for P. zeae, and on three hybrids for Meloidogyne spp. -
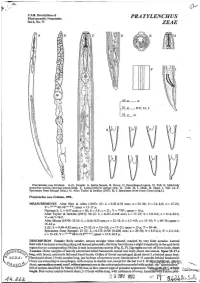
Pratylenchus Zeae
/p C.I.H. Descriptions of Plant-parasitic Nematodes PRATYLENCHUS Set 6, No. 77 ZEAE ' A D F V L 50 P A i5 ,B-F,H,I 25P ,G G Prutylenchus zeue Graham. A-G. Female. A. Entire female. B. Ovary. C. Oesophageal region. D. Tail. E. Mid-body ' transverse section showing lateral fields. F. Lateral field in surface view. G. Tails. H, I. Male. H. Head. I. Tail. (A-F. Specimens from Senegal (orig.). G. After Taylor & Jenkins (1957). H, I. Specimens from Ivory Coast (orig.).) PratyZemhus zeae Graham, 1951. MEASUREMENTS After Sher & Allen (1953): 99: L = 0.36-0.58 mm; a = 25-30; b = 5.4-8.0; c= 17-21; V = 26-43 68-763.4-6."; spear = 15-17 p. Neotype 9: L = 0.47 mm; a = 26; b = 5.9; c = 21 ; V = 30704;spear = 16 p. After Taylor & Jenkins (1957): 90 99: L = 0.413-0.639 mm; a = 17-25; b = 5.0-9.6; c = 11.2-24.1; v = 64.7-74.9. After Memy (1970): 25 99: L = 0.34-0.55 mm; a = 22-33; b = 3.3-4.9; c = 13-18; V = 69-74; spear = 15-18 p. 5 88: L = 0.40-0.42 mm; a = 27-32; b = 3.6-5.0; c = 17-21 ;spear = 15 p; T = 30-44. Specimens from Senegal: 25 99: L = 0.373-0.506 (0.428) mm; a = 20-30; b = 4.9-6.1 ; b' = 3.2-4.6; c = 15-19; V = 23-38 68.6-73.93-86.7; spear= 15.5-16.5 p. -

Plant-Parasitic Nematodes on Field Crops in South Africa
CORE Metadata, citation and similar papers at core.ac.uk Provided by Horizon / Pleins textes Fundam. appl. Nematol., 1992,15 (1), 7-14. Plant-parasitic nematodes on field crops in South Africa. 4. Groundnut Cheryl VENTER *, Dirk DE WAELE *+ and Cornelis F. VAN EEDEN ** .. Grain Crops Research Institute, Private Bag X1251, PotchefstTOom 2520, and .... Highveld Region, Privale Bag X804, Potchefstroom 2520, Republic of South Africa. Accepted for publication 20 November 1990. Summary - Sixteen groundnut fields, representative of the conditions prevailing in the groundnut-producing areas of the Orange Free State and Transvaal provinces ofSouth Africa were monitored. Four fields were monitored through the 1986/87 growing season and twelve through the 1987/88 growing season. Eighteen plant-parasitic nematode species were found. The predominant ectoparasites were Criconemella sphaerocephala and Paratrichodorus minor. The predominant endoparasites in both the roots and pods were Pratylenchus brachyurus and Ditylenchus destructor. Paratrichodorus lobatus, Scutellonema brachyurum, Xiphinema coomansi, X. vanderlindei, X. variabile, X. vitis, Longidorus pisi, Rotylenchus brevicaudatus, Rotylenchus incultus, R. triannulatus, R. unisexus, Helicotylenchus dihystera, Pracylenchus zeae and Rotylenchulus Pal"VUS were also found. In the soil, total populations of plant-parasitic nematodes increased or remained stable between planting and harvest in five fields, while those in eleven fields decreased. In the roots, total populations decreased between four weeks after planting and harvest in ail sixteen fields. In the pods, total populations increased or remained stable between eight weeks after planting and harvest in seven fields, while those in two fields decreased. Plant-parasitic nematodes were absent in the pods of six fields. C. sphaerocephala, P. -

Genus Pratylenchus Filipjev: Multientry and Monoentry Keys and Diagnostic Relationships (Nematoda: Tylenchida: Pratylenchidae)
© Zoological Institute, St.Petersburg, 2002 Genus Pratylenchus Filipjev: multientry and monoentry keys and diagnostic relationships (Nematoda: Tylenchida: Pratylenchidae) A.Y. Ryss Ryss, A.Y. 2002. Genus Pratylenchus Filipjev: multientry and monoentry keys and diag- nostic relationships (Nematoda: Tylenchida: Pratylenchidae). Zoosystematica Rossica, 10(2), 2001: 241-255. Tabular (multientry) key to Pratylenchus is presented, and functioning of the computer- ized multientry image-operating key developed on the basis of the stepwise computer diagnostic system BIKEY-PICKEY is described. Monoentry key to Pratylenchus is given, and diagnostic relationships are analysed with the routine taxonomic methods as well as with the use of BIKEY diagnostic system and by the cluster tree analysis using STATISTICA program package. The synonymy Pratylenchus scribneri Steiner in Sherbakoff & Stanley, 1943 = P. jordanensis Hashim, 1983, syn. n. is established. Con- clusion on the transition from amphimixis to parthenogenesis as one of the leading evolu- tionary factors for Pratylenchus is drawn. A.Y. Ryss, Zoological Institute, Russian Academy of Sciences, Universitetskaya nab. 1, St.Petersburg 199034, Russia. Identification of nematode species is difficult diagnostic system, and by the cluster tree analy- because of relative poverty and significant sis using STATISTICA program package intraspecific variability of diagnostic characters. (STATISTICA, 1995). The genus Pratylenchus Filipjev is an example of a group with large number of species (49 valid Material and the basic information sources species, more than 100 original descriptions) and complicated diagnostics. The genus has a world- The collections of the following institutions wide distribution and economic importance as were used in research: Zoological Institute, Rus- its species are the dangerous parasites of agri- sian Academy of Sciences; Institute for Nema- cultural crops. -

193 Molecular, Morphological and Thermal Characters of 19 Pratylenchus Spp. and Relatives Using the D3 Segment of the Nuclear Ls
MOLECULAR, MORPHOLOGICAL AND THERMAL CHARACTERS OF 19 PRATYLENCHUS SPP. AND RELATIVES USING THE D3 SEGMENT OF THE NUCLEAR LSU rRNA GENE Lynn K. Carta, Andrea M. Skantar, and Zafar A. Handoo USDA-ARS, Plant Sciences Institute, Nematology Lab, Beltsville, MD 20705, U.S.A. ABSTRACT Carta, L. K., A. M. Skantar, and Z. A. Handoo. 2001. Molecular, morphological and thermal charac- ters of 19 Pratylenchus spp. and relatives using the D3 segment of the nuclear LSU rRNA gene. Nem- atropica 31:195-209. Gene sequences are provided for the D3 segment of the large subunit rRNA gene in Pratylenchus agilis, P. hexincisus, P. teres, and P. zeae. They were aligned with the closest comparable previously pub- lished molecular sequences and evaluated with parsimony, distance and maximum-likelihood meth- ods. Different outgroups and more taxa in this study compared to a previous D3 tree resulted in improved phylogenetic resolution. Congruence of trees with thermal, vulval and lip characters was evaluated. A tropical clade of Pratylenchus with 2 lip annules was seen in all trees. Maximum-Parsimony and Quartet-Puzzling Maximum-Likelihood trees, with ambiguously-alignable positions excluded and Radopholus similis as an outgroup, had topologies congruent with species possessing 2, 3 or 4 lip annules. An updated sequence for Pratylenchus hexincisus indicated it was an outgroup of P. penetrans, P. arlingtoni, P. fallax and P. convallariae. Pratylenchus zeae was related to P. neglectus in a Neighbor-Join- ing tree, but was equivocal in others. The relatives of P. teres were P. neglectus and Hirschmanniella belli rather than morphometrically similar P. crenatus. -

Research/Investigación Competition Between Pratylenchus Zeaeand Meloidogyne Incognita on Sugarcane
RESEARCH/INVESTIGACIÓN COMPETITION BETWEEN PRATYLENCHUS ZEAE AND MELOIDOGYNE INCOGNITA ON SUGARCANE Lais Fernanda Fontana1, Claudia Regina Dias-Arieira*1, Danielle Mattei1, José Junior Severino2, Fabio Biela1, and Jailson de Oliveira Arieira2 1State University of Maringa, Agronomy Post Graduate, Maringa, PR, Brazil; 2State University of Maringa, Umuarama Regional Campus, Department of Agriculture, Umuarama, Paraná, Brazil. *Corresponding author: [email protected]. ABSTRACT Fontana, L. F., C. R. Dias-Arieira, D. Mattei, J. J. Severino, F. Biela, and J. O. Arieira. 2015. Competition between Pratylenchus zeae and Meloidogyne incognita on sugarcane. Nematropica 45:1-8. Mixed populations of nematodes involving sedentary endoparasites (Meloidogyne spp.) and the migratory endoparasite Pratylenchus zeae are frequent in sugarcane production areas, but details about the competition between these nematodes is not well known. This study aimed to evaluate the competition between M. incognita and P. zeae, as well as the effect of mixed populations on sugarcane. The study was divided into two experiments with four treatments. One experiment used an initial population of 1,000 P. zeae per plant and varied M. incognita inoculum levels from 0 to 4,000 eggs per plant. The other experiment consisted of an initial population density of 2,000 eggs of M. incognita and P. zeae levels ranging from 0 to 2,000 nematodes per plant. Ninety days after inoculation the increase in the initial population of one of the species caused a reduction in the reproduction of the other species although both species significantly increased their populations on sugarcane. With the same inoculum level, P. zeae showed greater reproductive capacity than M.