Grain Yield and Heterosis of Maize Hybrids Under Nematode Infested and Nematicide Treated Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathogenicity of Pratylenchus Hexincisus on Corn, Soybean, And
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1979 Pathogenicity of Pratylenchus hexincisus on corn, soybean, and tomato and population changes as influenced by hosts, temperature and soil type Mohammad Esmail Zirakparvar Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agricultural Science Commons, Agronomy and Crop Sciences Commons, and the Plant Pathology Commons Recommended Citation Zirakparvar, Mohammad Esmail, "Pathogenicity of Pratylenchus hexincisus on corn, soybean, and tomato and population changes as influenced by hosts, temperature and soil type" (1979). Retrospective Theses and Dissertations. 7260. https://lib.dr.iastate.edu/rtd/7260 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. -

Research/Investigación Aggressiveness Of
RESEARCH/INVESTIGACIÓN AGGRESSIVENESS OF PRATYLENCHUS BRACHYURUS TO SUGARCANE, COMPARED WITH KEY NEMATODE P. ZEAE Bruno Flávio Figueiredo Barbosa1*, Jaime Maia dos Santos¹, José Carlos Barbosa¹, Pedro Luiz Martins Soares1, Anderson Robert Ruas², Rafael Bernal de Carvalho¹ ¹Jaboticabal Unit, UNESP São Paulo State University, Department of Plant Protection, Jaboticabal, SP, 14884-900, Brazil. ²São Luiz College, Jaboticabal, SP, 14870-370, Brazil. The work is part of the doctorate thesis in Agronomy (Crop Production) of the first author. Author for correspondence: [email protected] ABSTRACT Barbosa, B. F. F., J. M. dos Santos, J. C. Barbosa, P. L. M. Soares, A. R. Ruas, R. B. de Carvalho. 2013. Aggressiveness of Pratylenchus brachyurus to the sugarcane, compared with key nematode P. zeae. Nematropica 43:119-130. Pratylenchus zeae, Meloidogyne javanica and M. incognita are considered key species of nematodes in sugarcane in Brazil, but P. brachyurus is also frequently found. This study was conducted to determine the aggressiveness of P. brachyurus compared with P. zeae to sugarcane. Plants were grown in pots (100 L) in an open area with initial inoculation of 10, 100, 1,000, 10,000 and 100,000/plant for P. brachyurus and P. zeae. The nematode inocula were from in vitro, carrot-cylinder cultures. Sampling was performed every 60 days until 300 days after inoculation. At harvest, we evaluated the population dynamics of the nematodes and plant growth characteristics. The population for the initial levels of 10 and 100,000 specimens/plant, for P. brachyurus and P. zeae at 300 days after inoculation were similar. This fact shows that, upon detection of nematodes in a certain place during the planting of sugarcane, the ratoon on this area should be treated so as to control populations of P. -

Theory Manual Course No. Pl. Path
NAVSARI AGRICULTURAL UNIVERSITY Theory Manual INTRODUCTORY PLANT NEMATOLOGY Course No. Pl. Path 2.2 (V Dean’s) nd 2 Semester B.Sc. (Hons.) Agri. PROF.R.R.PATEL, ASSISTANT PROFESSOR Dr.D.M.PATHAK, ASSOCIATE PROFESSOR Dr.R.R.WAGHUNDE, ASSISTANT PROFESSOR DEPARTMENT OF PLANT PATHOLOGY COLLEGE OF AGRICULTURE NAVSARI AGRICULTURAL UNIVERSITY BHARUCH 392012 1 GENERAL INTRODUCTION What are the nematodes? Nematodes are belongs to animal kingdom, they are triploblastic, unsegmented, bilateral symmetrical, pseudocoelomateandhaving well developed reproductive, nervous, excretoryand digestive system where as the circulatory and respiratory systems are absent but govern by the pseudocoelomic fluid. Plant Nematology: Nematology is a science deals with the study of morphology, taxonomy, classification, biology, symptomatology and management of {plant pathogenic} nematode (PPN). The word nematode is made up of two Greek words, Nema means thread like and eidos means form. The words Nematodes is derived from Greek words ‘Nema+oides’ meaning „Thread + form‟(thread like organism ) therefore, they also called threadworms. They are also known as roundworms because nematode body tubular is shape. The movement (serpentine) of nematodes like eel (marine fish), so also called them eelworm in U.K. and Nema in U.S.A. Roundworms by Zoologist Nematodes are a diverse group of organisms, which are found in many different environments. Approximately 50% of known nematode species are marine, 25% are free-living species found in soil or freshwater, 15% are parasites of animals, and 10% of known nematode species are parasites of plants (see figure at left). The study of nematodes has traditionally been viewed as three separate disciplines: (1) Helminthology dealing with the study of nematodes and other worms parasitic in vertebrates (mainly those of importance to human and veterinary medicine). -

The Effect of Pratylenchus Zeae on the Growth and Yield of Upland Rice
The effect of Pratylenchus zeae on the growth and yield of upland rice Richard A. PLOWRIGHT",Danilo MATUS**, Tin AUNG**and Twng-Wah MEW** * CAB International Institute of Parasitology, 395 a, Hatfield Road, St. Albans, Hertfordshire, AL4 OXU, UK and ** International Rice Research Institute, P. O. Box 933, Manila, Philippines. SUMMARY The root lesion nematode Pratylenchus zeae is widely distributed on upland rice but its economic importance has not been assessed. In a field trial, following a five month clean fallow, the ofcontrol P. zeae using carbofuran, increased the yieldof cv. Upl Ri-5 whilst the yield of cv. Kinandang Patong was unaffected. Pre-sowing soil population densities (Pi) of P. zeae were low (0-1 11 nematodes/lOOml soil) and there were no obvious symptoms of infectionduring early vegetativegrowth although the plant height of Upl Ri-5 was slightly reduced. At harvest the yield of treated plants was increased byO/O 13-29of that of untreated plants having a mean infection of 1 350 nematodedg root(P < 0.05). In the glasshouse the rate of growth and tilleringof cv. IR36 was significantly reduced with a highPi (630-3 O00 nematodesllO0 cm3 soil). Infected root systems were stunted and mean root fresh weight was reduced by 40-60%. Although infection reducedthe no. of spikeletslplant, these plants had a higher harvest index and consequently grain yield was unaffected. The relationship between yield and the population density of P. zeae at different crop growth stages, in the field indicates low tolerance and a high relative minimum yield of 65O/o. RESUMÉ Influence de Pratylenchus zeae sur la croissance et la récolte du riz de plateau Pratylenchus zeae est très répandusur le riz de plateau mais son importance n'a jamais été évaluée. -
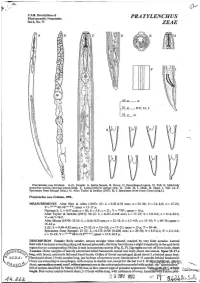
Pratylenchus Zeae
/p C.I.H. Descriptions of Plant-parasitic Nematodes PRATYLENCHUS Set 6, No. 77 ZEAE ' A D F V L 50 P A i5 ,B-F,H,I 25P ,G G Prutylenchus zeue Graham. A-G. Female. A. Entire female. B. Ovary. C. Oesophageal region. D. Tail. E. Mid-body ' transverse section showing lateral fields. F. Lateral field in surface view. G. Tails. H, I. Male. H. Head. I. Tail. (A-F. Specimens from Senegal (orig.). G. After Taylor & Jenkins (1957). H, I. Specimens from Ivory Coast (orig.).) PratyZemhus zeae Graham, 1951. MEASUREMENTS After Sher & Allen (1953): 99: L = 0.36-0.58 mm; a = 25-30; b = 5.4-8.0; c= 17-21; V = 26-43 68-763.4-6."; spear = 15-17 p. Neotype 9: L = 0.47 mm; a = 26; b = 5.9; c = 21 ; V = 30704;spear = 16 p. After Taylor & Jenkins (1957): 90 99: L = 0.413-0.639 mm; a = 17-25; b = 5.0-9.6; c = 11.2-24.1; v = 64.7-74.9. After Memy (1970): 25 99: L = 0.34-0.55 mm; a = 22-33; b = 3.3-4.9; c = 13-18; V = 69-74; spear = 15-18 p. 5 88: L = 0.40-0.42 mm; a = 27-32; b = 3.6-5.0; c = 17-21 ;spear = 15 p; T = 30-44. Specimens from Senegal: 25 99: L = 0.373-0.506 (0.428) mm; a = 20-30; b = 4.9-6.1 ; b' = 3.2-4.6; c = 15-19; V = 23-38 68.6-73.93-86.7; spear= 15.5-16.5 p. -

Plant-Parasitic Nematodes on Field Crops in South Africa
CORE Metadata, citation and similar papers at core.ac.uk Provided by Horizon / Pleins textes Fundam. appl. Nematol., 1992,15 (1), 7-14. Plant-parasitic nematodes on field crops in South Africa. 4. Groundnut Cheryl VENTER *, Dirk DE WAELE *+ and Cornelis F. VAN EEDEN ** .. Grain Crops Research Institute, Private Bag X1251, PotchefstTOom 2520, and .... Highveld Region, Privale Bag X804, Potchefstroom 2520, Republic of South Africa. Accepted for publication 20 November 1990. Summary - Sixteen groundnut fields, representative of the conditions prevailing in the groundnut-producing areas of the Orange Free State and Transvaal provinces ofSouth Africa were monitored. Four fields were monitored through the 1986/87 growing season and twelve through the 1987/88 growing season. Eighteen plant-parasitic nematode species were found. The predominant ectoparasites were Criconemella sphaerocephala and Paratrichodorus minor. The predominant endoparasites in both the roots and pods were Pratylenchus brachyurus and Ditylenchus destructor. Paratrichodorus lobatus, Scutellonema brachyurum, Xiphinema coomansi, X. vanderlindei, X. variabile, X. vitis, Longidorus pisi, Rotylenchus brevicaudatus, Rotylenchus incultus, R. triannulatus, R. unisexus, Helicotylenchus dihystera, Pracylenchus zeae and Rotylenchulus Pal"VUS were also found. In the soil, total populations of plant-parasitic nematodes increased or remained stable between planting and harvest in five fields, while those in eleven fields decreased. In the roots, total populations decreased between four weeks after planting and harvest in ail sixteen fields. In the pods, total populations increased or remained stable between eight weeks after planting and harvest in seven fields, while those in two fields decreased. Plant-parasitic nematodes were absent in the pods of six fields. C. sphaerocephala, P. -

Genus Pratylenchus Filipjev: Multientry and Monoentry Keys and Diagnostic Relationships (Nematoda: Tylenchida: Pratylenchidae)
© Zoological Institute, St.Petersburg, 2002 Genus Pratylenchus Filipjev: multientry and monoentry keys and diagnostic relationships (Nematoda: Tylenchida: Pratylenchidae) A.Y. Ryss Ryss, A.Y. 2002. Genus Pratylenchus Filipjev: multientry and monoentry keys and diag- nostic relationships (Nematoda: Tylenchida: Pratylenchidae). Zoosystematica Rossica, 10(2), 2001: 241-255. Tabular (multientry) key to Pratylenchus is presented, and functioning of the computer- ized multientry image-operating key developed on the basis of the stepwise computer diagnostic system BIKEY-PICKEY is described. Monoentry key to Pratylenchus is given, and diagnostic relationships are analysed with the routine taxonomic methods as well as with the use of BIKEY diagnostic system and by the cluster tree analysis using STATISTICA program package. The synonymy Pratylenchus scribneri Steiner in Sherbakoff & Stanley, 1943 = P. jordanensis Hashim, 1983, syn. n. is established. Con- clusion on the transition from amphimixis to parthenogenesis as one of the leading evolu- tionary factors for Pratylenchus is drawn. A.Y. Ryss, Zoological Institute, Russian Academy of Sciences, Universitetskaya nab. 1, St.Petersburg 199034, Russia. Identification of nematode species is difficult diagnostic system, and by the cluster tree analy- because of relative poverty and significant sis using STATISTICA program package intraspecific variability of diagnostic characters. (STATISTICA, 1995). The genus Pratylenchus Filipjev is an example of a group with large number of species (49 valid Material and the basic information sources species, more than 100 original descriptions) and complicated diagnostics. The genus has a world- The collections of the following institutions wide distribution and economic importance as were used in research: Zoological Institute, Rus- its species are the dangerous parasites of agri- sian Academy of Sciences; Institute for Nema- cultural crops. -

Research/Investigación Competition Between Pratylenchus Zeaeand Meloidogyne Incognita on Sugarcane
RESEARCH/INVESTIGACIÓN COMPETITION BETWEEN PRATYLENCHUS ZEAE AND MELOIDOGYNE INCOGNITA ON SUGARCANE Lais Fernanda Fontana1, Claudia Regina Dias-Arieira*1, Danielle Mattei1, José Junior Severino2, Fabio Biela1, and Jailson de Oliveira Arieira2 1State University of Maringa, Agronomy Post Graduate, Maringa, PR, Brazil; 2State University of Maringa, Umuarama Regional Campus, Department of Agriculture, Umuarama, Paraná, Brazil. *Corresponding author: [email protected]. ABSTRACT Fontana, L. F., C. R. Dias-Arieira, D. Mattei, J. J. Severino, F. Biela, and J. O. Arieira. 2015. Competition between Pratylenchus zeae and Meloidogyne incognita on sugarcane. Nematropica 45:1-8. Mixed populations of nematodes involving sedentary endoparasites (Meloidogyne spp.) and the migratory endoparasite Pratylenchus zeae are frequent in sugarcane production areas, but details about the competition between these nematodes is not well known. This study aimed to evaluate the competition between M. incognita and P. zeae, as well as the effect of mixed populations on sugarcane. The study was divided into two experiments with four treatments. One experiment used an initial population of 1,000 P. zeae per plant and varied M. incognita inoculum levels from 0 to 4,000 eggs per plant. The other experiment consisted of an initial population density of 2,000 eggs of M. incognita and P. zeae levels ranging from 0 to 2,000 nematodes per plant. Ninety days after inoculation the increase in the initial population of one of the species caused a reduction in the reproduction of the other species although both species significantly increased their populations on sugarcane. With the same inoculum level, P. zeae showed greater reproductive capacity than M. -

Research Note First Report of the Lesion Nematodes
©2018 Institute of Parasitology, SAS, Košice DOI 10.1515/helm-2017-0053 HELMINTHOLOGIA, 55, 1: 88 – 94, 2018 Research Note First report of the lesion nematodes: Pratylenchus brachyurus and Pratylenchus delattrei on tomato (Solanum lycopersicum L.) plants in Cape Verde Ł. FLIS1*, R. DOBOSZ2, K. RYBARCZYK-MYDŁOWSKA1, B. WASILEWSKA-NASCIMENTO3, M. KUBICZ1, G. WINISZEWSKA1 1Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warszawa, Poland, E-mail: *lfl [email protected], [email protected]; [email protected]; [email protected]; 2Institute of Plant Protection-National Research Institute, Węgorka 20, 60-318, Poznań, Poland, E-mail: [email protected]; 3University of Cape Verde, Palmarejo, CP 279-Praia, Republic of Cape Verde, E-mail: [email protected] Article info Summary Received July 3, 2017 Roots of Solanum lycopersicum L. were collected in growing season of year 2015, on the island of Accepted September 28, 2017 Santiago in Cape Verde. Morphological, morphometric and molecular (18S rDNA and 28S rDNA) studies revealed the presence of Pratylenchus brachyurus and P. delattrei in root systems and root zones of tomato plants. To our knowledge, this is the fi rst record of the occurrence of these nematode species in Cape Verde. Keywords: Cape Verde; new geographic record; Pratylenchus brachyurus; Pratylenchus delattrei; 18S rDNA; 28S rDNA Introduction migratory endoparasites move within host root tissues causing necrosis and creating wounds, thus providing openings for soil- Tomato (Solanum lycopersicum L.) is one of the vegetable crops borne plant pathogens to enter and cause disease. In tropical and most widely grown on irrigated land in the Republic of Cape Verde. -

Taxonomïc Studies on the Genus Pratylenchus (Nematoda)
TAXONOMÏC STUDIES ON THE GENUS PRATYLENCHUS (NEMATODA) (Taxonomische onderzoekingen aan het nemaiodengeslacht pratylenchusj DOOR P. A. A. LOOF (Plantenziektenkundige Dienst) Nr 39 (I960) Published also in: T. PI. ziekten 66 (I960) : 29-90. CONTENTS Preface 6 A. General and experimental section 7 I. Introduction 7 H. Taxonomiecharacter s 9 lu The identity of Tylenchus pratensis DE MAN 13 IV. The identity of Tylenchusgulosus KÜHN 21 V. The identity of Aphelenchus neglectus RENSCH 23 VI. Taxonomy ofPratylenchus minyus SWSM. &ALLE N 28 VII. The taxonomiestatu s ofPratylenchus coffeae (ZIMMERMANN) 34 VIII. Some remarksconcernin g thedeterminatio n of males 38 B. Systematic section 40 IX. Validspecie s 40 Key -40 Descriptions 41 X. Species inquirendae 59 XI. Synonymized and transferred species 60 Samenvatting 62 Literature cited 63 PREFACE The following studies on the nematode genus Pratylenchus form a review supplementary to the pioneer work by SHER & ALLEN (1953), who put the taxonomy of the genus on a sound basis. For information not contained in the following pages, the reader is referred to their paper. Many nematologists have assisted the author with helpful criticism and sugges tions, information on various points or with nematode material. Sincere thanks are offered to Prof. Dr. M. W. ALLEN (Berkeley, California, U.S.A.), Dr. P. BOVIEN (Lyngby, Denmark), Dr. J. R. CHRISTIE (Gainesville, Florida, U.S.A.), Dr. H. GOFFART (Münster, Germany), Dr. W. R. JENKINS (College Park, Maryland, U.S.A.), Mr. J. KRADEL (Kleinmachnow, Germany), Prof. Dr. H. A. KREIS (Bern, Switzerland), Dr. D. PAETZOLD (Halle, Germany), Prof. Dr. B. RENSCH (Münster, Germany), Drs. -

JOURNAL of NEMATOLOGY a COI DNA Barcoding Survey of Pratylenchus Species in the Great Plains Region of North America
JOURNAL OF NEMATOLOGY Article | DOI: 10.21307/jofnem-2019-081 e2019-81 | Vol. 51 A COI DNA barcoding survey of Pratylenchus species in the Great Plains Region of North America Mehmet Ozbayrak,1 Tim Todd,2 Timothy Harris,1 Rebecca Higgins,1 Kirsten Powers,1 Peter Mullin,1 Abstract 1 1 Lisa Sutton and Thomas Powers * Pratylenchus species are among the most common plant parasitic 1Department of Plant Pathology, nematodes in the Great Plains Region of North America. Our goal University of Nebraska-Lincoln, was to survey Pratylenchus species diversity across the Great Plains Lincoln, NE, 68583-0722. region using a mitochondrial COI DNA barcode. The objectives were to (i) determine species boundaries of the common Pratylen- 2 Department of Plant Pathology, chus species within the region, (ii) assess the host associations of Kansas State University, the barcoded Pratylenchus specimens, and (iii) determine Pratylen- Manhattan, KS, 66502. chus distribution patterns throughout the region. A total of 860 soil *E-mail: [email protected] samples, primarily associated with eight major crops, were collect- ed from Colorado, Kansas, Montana, Nebraska, North Dakota, and This paper was edited by Wyoming. From this total, 246 soil samples provided the majority of Erik J. Ragsdale. 915 individual nematode specimens that were amplified by PCR and Received for publication May 24, sequenced for a 727 to 739 bp region of COI. Maximum likelihood, 2019. neighbor-joining, and Bayesian phylogenetic trees all recognized 19 distinct and well-supported haplotype groups. The most common and widespread haplotype group, representing 53% of all specimens was P. neglectus, detected from 178 fields in 100 counties and as- sociated with fields growing wheat, corn, dry beans, barley, alfalfa, sugar beets, potatoes, and a vineyard. -

FINAL THESIS Submission Newest
Genetic studies and recurrent selection for nematode resistance in maize Frank Kagoda BSc. Agric. (Hons), MSc. Crop Science (Makerere University, Kampala, Uganda) A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) in Plant Breeding African Centre for Crop Improvement School of Agricultural Sciences and Agribusiness Faculty of Science and Agriculture University of KwaZulu Natal, Pietermaritzburg, South Africa December 2010 General abstract Plant-parasitic nematodes cause grain yield loss in maize. The most important genera of plant-parasitic nematodes demonstrated to be of economic importance to maize are Pratylenchus spp., Meloidogyne spp. and Heterodera spp. In Uganda, the most prevalent species are Pratylenchus zeae and Meloidogyne spp. The current study was initiated with the following objectives: (i) assessing farmers’ awareness of maize nematodes, other maize production constraints, and desirable agronomic traits; (ii) assessing the efficiency of sterile carrot discs for mass culturing of Pratylenchus zeae; (iii) Characterising the inheritance of nematode resistance in maize, through, estimation of the general combining ability (GCA) of various parents, and the specific combining ability (SCA) of a parent in a cross with another parent; and through determining the contribution of cytoplasmic effects to inheritance of resistance to nematodes; and (iv) determine the level of nematode resistance among F 1 hybrids and estimate grain yield, heterosis and yield losses associated with maize hybrids under nematode infestation; (v) comparing the gains in nematode resistance and grain yield obtained following two cycles of S 1 progeny recurrent selection in three tropical maize populations. In the participatory rural appraisal, data were collected from 120 households in two maize- growing districts.