December 2020 E-Tips Solid Tumor Rules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Follicular Lymphoma
Follicular Lymphoma What is follicular lymphoma? Let us explain it to you. www.anticancerfund.org www.esmo.org ESMO/ACF Patient Guide Series based on the ESMO Clinical Practice Guidelines FOLLICULAR LYMPHOMA: A GUIDE FOR PATIENTS PATIENT INFORMATION BASED ON ESMO CLINICAL PRACTICE GUIDELINES This guide for patients has been prepared by the Anticancer Fund as a service to patients, to help patients and their relatives better understand the nature of follicular lymphoma and appreciate the best treatment choices available according to the subtype of follicular lymphoma. We recommend that patients ask their doctors about what tests or types of treatments are needed for their type and stage of disease. The medical information described in this document is based on the clinical practice guidelines of the European Society for Medical Oncology (ESMO) for the management of newly diagnosed and relapsed follicular lymphoma. This guide for patients has been produced in collaboration with ESMO and is disseminated with the permission of ESMO. It has been written by a medical doctor and reviewed by two oncologists from ESMO including the lead author of the clinical practice guidelines for professionals, as well as two oncology nurses from the European Oncology Nursing Society (EONS). It has also been reviewed by patient representatives from ESMO’s Cancer Patient Working Group. More information about the Anticancer Fund: www.anticancerfund.org More information about the European Society for Medical Oncology: www.esmo.org For words marked with an asterisk, a definition is provided at the end of the document. Follicular Lymphoma: a guide for patients - Information based on ESMO Clinical Practice Guidelines – v.2014.1 Page 1 This document is provided by the Anticancer Fund with the permission of ESMO. -

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

Variants of Acinar Adenocarcinoma of the Prostate Mimicking Benign Conditions Peter a Humphrey
Modern Pathology (2018) 31, S64–S70 S64 © 2018 USCAP, Inc All rights reserved 0893-3952/18 $32.00 Variants of acinar adenocarcinoma of the prostate mimicking benign conditions Peter A Humphrey Department of Pathology, Yale School of Medicine, New Haven, CT, USA Histological variants of acinar adenocarcinoma of the prostate may be of significance due to difficulty in diagnosis or due to differences in prognosis compared to usual acinar adenocarcinoma. The 2016 World Health Organization classification of acinar adenocarcinoma includes four variants that are deceptively benign in histological appearance, such that a misdiagnosis of a benign condition may be made. These four variants are atrophic pattern adenocarcinoma, pseudohyperplastic adenocarcinoma, microcystic adenocarcinoma, and foamy gland adenocarcinoma. They differ from usual small acinar adenocarcinoma in architectural glandular structure and/or cytoplasmic and nuclear alterations. The variants are often admixed, in variable proportions, with usual small acinar adenocarcinoma that is often Gleason pattern 3 but may be high-grade pattern 4 in a minority of cases. Atrophic pattern adenocarcinoma can be identified in a sporadic setting or after radiation or hormonal therapy. This variant is characterized by cytoplasmic volume loss and can resemble benign glandular atrophy, an extremely common benign process in the prostate. The glands of pseudohyperplastic adenocarcinoma simulate usual epithelial hyperplasia, with gland complexity that is not typical of small acinar adenocarcinoma. These complex growth configurations include papillary infoldings, luminal undulations, and branching. Microcystic adenocarcinoma is characterized by cystic dilation of prostatic glands to a size that is much more commonly observed in cystic change in benign prostatic glands. Finally, the cells in foamy gland adenocarcinoma display cytoplasmic vacuolization and nuclear pyknosis, features that can found in benign glands and macrophages. -

Supplementary Table 1) Immunohistochemical Protocol and Antibody Information Antibody Company Clone/# Clonality Dilution Incubat
H. Reis et al: Differential proteomic and tissue expression analyses identify valuable diagnostic biomarkers of hepatocellular differentiation and hepatoid adenocarcinomas Suppl ementary Table 1) Immunohistochemical protocol and antibody information Antibody Company Clone/# Clonality Dilution Incubation Antigen retrieval Detection ABAT Abcam EPR4433 mono 1/3000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP ACAA2 Abcam EPR6733 mono 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP ACADM Abcam EPR3708 mono 1/3000 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP ADH1B Abcam 4F12 mono 1/12.000 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP Arginase1 Abcam EPR6672(B) mono 1/1000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP BHMT Abcam EPR6782 mono 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP FABP1 Acris AIV\09011PU-S mono 1/15.000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HAOX1 Acris AP18044PU-N poly 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HepPar1 Dako OCH1E5 mono 1/800 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HMGCS2 Abcam Ab104807 poly 1/50 60 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP H. Reis et al: Differential proteomic and tissue expression analyses identify valuable diagnostic biomarkers of hepatocellular differentiation and hepatoid adenocarcinomas Supplementary Table 2) Composition of the non-liver tumor TMA Diagnosis n % ADC 11 2.9 ADC Adenocarcinoma of the lung Carc. -

1 Effective January 1, 2018 ICD‐O‐3 Codes, Behaviors and Terms Are Site‐Specific Alpha Order Last Updat
Effective January 1, 2018 ICD‐O‐3 codes, behaviors and terms are site‐specific Alpha Order Last updated 8/22/18 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New Term 8551/3 Acinar adenocarcinoma (C34. _) Y Lung primaries diagnosed prior to 1/1/2018 use code 8550/3 For prostate (all years) see 8140/3 New Term 8140/3 Acinar adenocarcinoma (C61.9 ONLY) Y For prostate only, do not use 8550/3 New Term 8572/3 Acinar adenocarcinoma, sarcomatoid (C61.9) Y New Term 8550/3 Acinar cell carcinoma Y Excludes C61.9‐ see 8140/3 New Term 8316/3 Acquired cystic disease‐associated renal cell carcinoma (RCC) Y (C64.9) New 8158/1 ACTH‐producing tumor N code/term New Term 8574/3 Adenocarcinoma admixed with neuroendocrine carcinoma (C53. _) Y Behavior 8253/2 Adenocarcinoma in situ, mucinous (C34. _) Y Important note: lung Code/term primaries ONLY: For cases diagnosed 1/1/2018 forward do not use code 8480 (mucinous adenocarcinoma) for in‐ situ adenocarcinoma, mucinous or invasive mucinous adenocarcinoma. 1 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code Behavior 8250/2 Adenocarcinoma in situ, non‐mucinous (C34. _) Y code/term New Term 9110/3 Adenocarcinoma of rete ovarii (C56.9) Y New 8163/3 Adenocarcinoma, pancreatobiliary‐type (C24.1) Y Cases diagnosed prior to code/term 1/1/2018 use code 8255/3 Behavior 8983/3 Adenomyoepithelioma with carcinoma (C50. _) Y Code/term New Term 8620/3 Adult granulosa cell tumor (C56.9 ONLY) N Not reportable for 2018 cases New Term 9401/3 Anaplastic astrocytoma, IDH‐mutant (C71. -
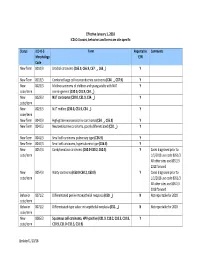
Updated 1/10/18 Effective January 1, 2018 ICD‐O‐3 Codes, Behaviors and Terms Are Site‐Specific Status IC
Effective January 1, 2018 ICD‐O‐3 codes, behaviors and terms are site‐specific Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New Term 8010/3 Urachal carcinoma (C65.9, C66.9, C67. _, C68._) Y New Term 8013/3 Combined large cell neuroendocrine carcinoma (C34. _, C37.9) Y New 8023/3 Midline carcinoma of children and young adults with NUT Y code/term rearrangement (C30.0, C31.9, C34. _) New 8023/3 NUT carcinoma (C30.0, C31.9, C34. _) Y code/term New 8023/3 NUT midline (C30.0, C31.9, C34. _) Y code/term New Term 8041/3 High‐grade neuroendocrine carcinoma (C54. _, C55.9) Y New Term 8041/3 Neuroendocrine carcinoma, poorly differentiated (C50. _) Y New Term 8041/3 Small cell carcinoma pulmonary type (C56.9) Y New Term 8044/3 Small cell carcinoma, hypercalcemic type (C56.9) Y New 8054/3 Condylomatous carcinoma (C60.0‐C60.2, C60.9) Y Cases diagnosed prior to code/term 1/1/2018 use code 8051/3 All other sites use 8051/3 2018 forward New 8054/3 Warty carcinoma (C60.0‐C60.2, C60.9) Y Cases diagnosed prior to code/term 1/1/2018 use code 8051/3 All other sites use 8051/3 2018 forward Behavior 8071/2 Differentiated penile intraepithelial neoplasia (C60. _) N Not reportable for 2018 code/term Behavior 8071/2 Differentiated‐type vulvar intraepithelial neoplasia (C51. _) N Not reportable for 2018 code/term New 8085/3 Squamous cell carcinoma, HPV‐positive (C01.9, C10.2, C10.3, C10.8, Y code/term C10.9, C31.0–C31.3, C31.9) Updated 1/10/18 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New 8086/3 Squamous cell carcinoma, HPV‐negative (C01.9, C10.2, C10.3, Y code/term C10.8, C10.9, C31.0–C31.3, C31.9) New Term 8120/3 Clear cell (glycogen‐rich) urothelial carcinoma (C65.9, C66.9, C67. -

List of Acceptable TCGA Tumor Types*
List of Acceptable TCGA Tumor Types* Acute Myeloid Leukemia Head and Neck Squamous ** Ovarian Carcinoma** Thyroid Papillary Carcinoma** Squamous Cell Carcinoma Serous Cystadenocarcinoma Papillary, Usual Type (Papillary, NOS) Bladder Urothelial Carcinoma Squamous Cell Carcinoma, Spindle Cell Variant Serous carcinoma Papillary, Follicular Variant (99% follicular patterned) Muscle invasive urothelial carcinoma (PT2 or above) Squamous Cell Carcinoma, Basaloid Type Serous adenocarcinoma Papillary, Tall cell Variant (50% tall cell features) Papillary Serous carcinoma Papillary, Other Breast Invasive Carcinoma Hepatocellular Carcinoma Papillary Serous cystoadenocarcinoma Infiltrating Ductal Carcinoma** Hepatocellular Carcinoma, NOS Serous Papillary carcinoma Rare Tumor Studies Infiltrating Lobular Carcinoma Fibrolamellar Hepatocellular Carcinoma Serous Papillary cystoadenocarcinoma Medullary Carcinoma Hepatocholangiocarcinoma (mixed) Serous Papillary adenocarcinoma Adrenocortcal Tumors*** Mucinous Carcinoma Adrenocortical carcinoma - Usual Type Metaplastic Carcinoma Kidney Adenocarcinoma Pancreatic Adenocarcinoma Adrenocortical carcinoma - Oncocytic Type Mixed (with Ductal) Histology*** Clear Cell Renal Cell Carcinoma** Adenocarcinoma, ductal type Adrenocortical carcinoma - Myxoid Type Other Papillary Renal Cell Carcinoma Colloid (mucinous non-cystic) Carcinoma Hepatoid Carcinoma Chromophobe Kidney*** Cervical Cancer Lower Grade Glioma** Medullary Carcinoma Cervical Squamous Cell Carcinoma Astrocytoma, Grade II Signet Ring Cell Carcinoma Mesothelioma -

About Mastectomy and Sentinel Lymph Node Biopsy
All About Mastectomy and Sentinel Lymph Node Biopsy One of the most important goals of Moffitt Cancer Center is to provide you with quality patient care through education, research and patient care. The following information has been developed to help you understand the mastectomy and sentinel lymph node biopsy procedures for which you have been scheduled. Members of your health care team will review this information with you and answer any questions that you may have. Definitions: Mastectomy – Removal of the entire breast but not the muscles underneath. Lymph Nodes - Small bean shaped glands found in the armpit. They remove waste and fluids from the arm and breast and help fight infection. They are also call lymph glands. Sentinel Lymph Node - The first place breast cancer may metastasize (or spread) is to lymph nodes in the axilla (underarm area) on the side of the body where the cancer is. The first lymph node(s) are referred to as the sentinel lymph node(s) or the “gate keepers”. These nodes are first biopsied to determine if the cancer has spread. If cancer cells are not found in the sentinel lymph node(s) that were removed, it will not be necessary to remove the remaining lymph nodes in the arm pit. Sentinel Lymph Node Mapping and Biopsy - This procedure is a two step process. Performing the sentinel lymph node biopsy requires a small incision in your axilla. In the first step, the doctor injects a radioactive substance and/ or blue dye in the area around the tumor. Lymphatic channels (like blood vessels) carry these materials to the sentinel lymph node. -

Two Cases of Solitary Fibrous Tumor Involving Urinary Bladder and a Review of the Literature
Hindawi Publishing Corporation Case Reports in Urology Volume 2016, Article ID 5145789, 5 pages http://dx.doi.org/10.1155/2016/5145789 Case Report Two Cases of Solitary Fibrous Tumor Involving Urinary Bladder and a Review of the Literature Eduardo Yukio Tanaka,1,2 Vitor Bonadia Buonfiglio,1,2 Joao Padua Manzano,1,2 Renée Zon Filippi,2 and Marcus Vinicius Sadi1,2 1 Federal University of Sao˜ Paulo, Sao˜ Paulo, SP, Brazil 2Hospital Israelita Albert Einstein, Sao˜ Paulo, SP, Brazil Correspondence should be addressed to Eduardo Yukio Tanaka; du [email protected] Received 12 June 2016; Revised 5 September 2016; Accepted 8 September 2016 Academic Editor: Fumitaka Koga Copyright © 2016 Eduardo Yukio Tanaka et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Solitary fibrous tumor (SFT) is a rare neoplasia of mesenchymal origin, initially described in visceral pleura and lately discovered to have ubiquitous distribution. SFT of the urogenital tract is uncommon and appears to have similar morphologic features and biologic behaviors as SFTs found elsewhere. We present two new cases of SFT of the bladder and review 22 similar cases published in the literature. Due to the general indolent behavior of these lesions, a complete but organ sparing surgical excision should be considered when technically feasible. Therefore, proper identification and characterization of SFT through morphological and immunohistochemical criteria on biopsy specimens are mandatory in the differential diagnosis from other more aggressive spindle- cell tumors, thus avoiding unnecessary radical surgery. -

Consensus Guideline on the Management of the Axilla in Patients with Invasive/In-Situ Breast Cancer
- Official Statement - Consensus Guideline on the Management of the Axilla in Patients With Invasive/In-Situ Breast Cancer Purpose To outline the management of the axilla for patients with invasive and in-situ breast cancer. Associated ASBrS Guidelines or Quality Measures 1. Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients – Revised November 25, 2014 2. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients – Approved November 25, 2014 3. Quality Measure: Sentinel Lymph Node Biopsy for Invasive Breast Cancer – Approved November 4, 2010 4. Prior Position Statement: Management of the Axilla in Patients With Invasive Breast Cancer – Approved August 31, 2011 Methods A literature review inclusive of recent randomized controlled trials evaluating the use of sentinel lymph node surgery and axillary lymph node dissection for invasive and in-situ breast cancer as well as the pathologic review of sentinel lymph nodes and indications for axillary radiation was performed. This is not a complete systematic review but rather, a comprehensive review of recent relevant literature. A focused review of non-randomized controlled trials was then performed to develop consensus guidance on management of the axilla in scenarios where randomized controlled trials data is lacking. The ASBrS Research Committee developed a consensus document, which was reviewed and approved by the ASBrS Board of Directors. Summary of Data Reviewed Recommendations Based on Randomized Controlled -

Labinvest201321.Pdf
190A ANNUAL MEETING ABSTRACTS Sensitivity, specificity, positive/negative predictive value, and the odds ratio of the SCC (0/33) by FISH. No difference of overall survival time was observed between 2 markers were summarized in Table 1. There was no difference in p16 expression amplified and non-amplified groups in EAC patients. between the DG and control group. Conclusions: ALK amplification was present in 7% of EAC cases, but ALK Table 1. Summary of sensitivity, specificity, PPV, NPV, and OR. rearrangement and expression was not present. No ALK amplification, rearrangement Sensitivity (%) Specificity (%) PPV (%) NPV (%) OR and expression were detected in SCC. ALK amplification is not associated with EAC beta-catenin 92.3 76.9 80.0 90.9 40.0 patients’ overall survival. c-met 69.2 61.5 64.3 66.7 3.6 Either 100.0 53.8 68.4 100.0 13.9* Both 61.5 92.3 88.9 70.6 19.2 785 Mitosis-Specific Marker PHH3 Immunostain Is a More Sensitive NPV, negative predictive value; PPV, positive predictive value; OR, odds ratio. *, estimated OR. and Efficient Method To Evaluate the Mitotic Activity in Gastrointestinal Conclusions: The combination of c-met and beta-catenin provides a relatively specific Stromal Tumor (GIST) panel to identify patients with BE who are at increased risk for future development S Zhu, F Lin, ZE Chen. Geisinger Medical Center, Danville, PA. of dysplasia. Background: Mitotic activity is an important prognostic factor in GIST. The accurate identification of mitotic figures on the H&E stained slides could be challenging due to 783 Microfibrillar Associated Protein 5 (MFAP 5): A Marker for processing artifact, degeneration, apoptosis, or lymphocytic infiltration. -

Application of Preoperative Computed Tomographic Lymphography For
Wen et al. BMC Surg (2021) 21:187 https://doi.org/10.1186/s12893-021-01190-7 RESEARCH ARTICLE Open Access Application of preoperative computed tomographic lymphography for precise sentinel lymph node biopsy in breast cancer patients Shishuai Wen1,2,3†, Yiran Liang1†, Xiaoli Kong1, Baofeng Liu4, Tingting Ma1, Yeqing Zhou1, Liyu Jiang1, Xiaoyan Li1 and Qifeng Yang1,5,6* Abstract Background: In light of the extensive application of sentinel lymph node biopsy (SLNB) in clinically node-negative breast cancer patients and the recently investigated failure of SLNB after lumpectomy, it has become important to explore methods for preoperative mapping of sentinel lymph nodes (SLNs) and their lymphatics to direct precise SLNB and improve the identifcation rate of SLNs. Methods: Twenty-seven patients with suspected breast cancer based on the results of the clinical examination and imaging were enrolled in the study. Computed tomographic lymphography (CTLG) followed by CT three-dimensional reconstruction was performed to determine the localization of SLNs and lymphatics on the body surface preopera- tively. Intraoperatively combined staining with methylene blue and indocyanine green was used to evaluate the accuracy and feasibility of CTLG. Results: SLNs and lymphatics from the breast were identifed using CTLG in all patients, and preoperative SLNs and lymphatics localization on the body surface showed a signifcant role in the selection of operative incision and injec- tion points. The accuracy rate of SLN and lymphatic detection by CTLG was 92.6% compared with intraoperatively combined staining. Moreover, preoperative CTLG performed well in SLN number detection, and the accuracy rate was 95.2%. Conclusion: We evaluate the procedure and application of preoperative CTLG in the superfcial localization of SLNs and lymphatics, which may lead to a decreased incidence of cutting of the lymphatics of SLNs and consequently more rapid and accurate SLN detection.