Two Cases of Solitary Fibrous Tumor Involving Urinary Bladder and a Review of the Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

Variants of Acinar Adenocarcinoma of the Prostate Mimicking Benign Conditions Peter a Humphrey
Modern Pathology (2018) 31, S64–S70 S64 © 2018 USCAP, Inc All rights reserved 0893-3952/18 $32.00 Variants of acinar adenocarcinoma of the prostate mimicking benign conditions Peter A Humphrey Department of Pathology, Yale School of Medicine, New Haven, CT, USA Histological variants of acinar adenocarcinoma of the prostate may be of significance due to difficulty in diagnosis or due to differences in prognosis compared to usual acinar adenocarcinoma. The 2016 World Health Organization classification of acinar adenocarcinoma includes four variants that are deceptively benign in histological appearance, such that a misdiagnosis of a benign condition may be made. These four variants are atrophic pattern adenocarcinoma, pseudohyperplastic adenocarcinoma, microcystic adenocarcinoma, and foamy gland adenocarcinoma. They differ from usual small acinar adenocarcinoma in architectural glandular structure and/or cytoplasmic and nuclear alterations. The variants are often admixed, in variable proportions, with usual small acinar adenocarcinoma that is often Gleason pattern 3 but may be high-grade pattern 4 in a minority of cases. Atrophic pattern adenocarcinoma can be identified in a sporadic setting or after radiation or hormonal therapy. This variant is characterized by cytoplasmic volume loss and can resemble benign glandular atrophy, an extremely common benign process in the prostate. The glands of pseudohyperplastic adenocarcinoma simulate usual epithelial hyperplasia, with gland complexity that is not typical of small acinar adenocarcinoma. These complex growth configurations include papillary infoldings, luminal undulations, and branching. Microcystic adenocarcinoma is characterized by cystic dilation of prostatic glands to a size that is much more commonly observed in cystic change in benign prostatic glands. Finally, the cells in foamy gland adenocarcinoma display cytoplasmic vacuolization and nuclear pyknosis, features that can found in benign glands and macrophages. -

Supplementary Table 1) Immunohistochemical Protocol and Antibody Information Antibody Company Clone/# Clonality Dilution Incubat
H. Reis et al: Differential proteomic and tissue expression analyses identify valuable diagnostic biomarkers of hepatocellular differentiation and hepatoid adenocarcinomas Suppl ementary Table 1) Immunohistochemical protocol and antibody information Antibody Company Clone/# Clonality Dilution Incubation Antigen retrieval Detection ABAT Abcam EPR4433 mono 1/3000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP ACAA2 Abcam EPR6733 mono 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP ACADM Abcam EPR3708 mono 1/3000 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP ADH1B Abcam 4F12 mono 1/12.000 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP Arginase1 Abcam EPR6672(B) mono 1/1000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP BHMT Abcam EPR6782 mono 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP FABP1 Acris AIV\09011PU-S mono 1/15.000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HAOX1 Acris AP18044PU-N poly 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HepPar1 Dako OCH1E5 mono 1/800 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HMGCS2 Abcam Ab104807 poly 1/50 60 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP H. Reis et al: Differential proteomic and tissue expression analyses identify valuable diagnostic biomarkers of hepatocellular differentiation and hepatoid adenocarcinomas Supplementary Table 2) Composition of the non-liver tumor TMA Diagnosis n % ADC 11 2.9 ADC Adenocarcinoma of the lung Carc. -

1 Effective January 1, 2018 ICD‐O‐3 Codes, Behaviors and Terms Are Site‐Specific Alpha Order Last Updat
Effective January 1, 2018 ICD‐O‐3 codes, behaviors and terms are site‐specific Alpha Order Last updated 8/22/18 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New Term 8551/3 Acinar adenocarcinoma (C34. _) Y Lung primaries diagnosed prior to 1/1/2018 use code 8550/3 For prostate (all years) see 8140/3 New Term 8140/3 Acinar adenocarcinoma (C61.9 ONLY) Y For prostate only, do not use 8550/3 New Term 8572/3 Acinar adenocarcinoma, sarcomatoid (C61.9) Y New Term 8550/3 Acinar cell carcinoma Y Excludes C61.9‐ see 8140/3 New Term 8316/3 Acquired cystic disease‐associated renal cell carcinoma (RCC) Y (C64.9) New 8158/1 ACTH‐producing tumor N code/term New Term 8574/3 Adenocarcinoma admixed with neuroendocrine carcinoma (C53. _) Y Behavior 8253/2 Adenocarcinoma in situ, mucinous (C34. _) Y Important note: lung Code/term primaries ONLY: For cases diagnosed 1/1/2018 forward do not use code 8480 (mucinous adenocarcinoma) for in‐ situ adenocarcinoma, mucinous or invasive mucinous adenocarcinoma. 1 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code Behavior 8250/2 Adenocarcinoma in situ, non‐mucinous (C34. _) Y code/term New Term 9110/3 Adenocarcinoma of rete ovarii (C56.9) Y New 8163/3 Adenocarcinoma, pancreatobiliary‐type (C24.1) Y Cases diagnosed prior to code/term 1/1/2018 use code 8255/3 Behavior 8983/3 Adenomyoepithelioma with carcinoma (C50. _) Y Code/term New Term 8620/3 Adult granulosa cell tumor (C56.9 ONLY) N Not reportable for 2018 cases New Term 9401/3 Anaplastic astrocytoma, IDH‐mutant (C71. -

December 2020 E-Tips Solid Tumor Rules
New Jersey State Cancer Registry December 2020 E-Tips Cancer Epidemiology Services http://www.nj.gov/health/ces (609) 633-0500 Solid Tumor Rules: December 2020 Update ICD-0-3.2 changes have also been added to applicable site modules. Most changes are minor: terminology, additional definitions, new notes and examples. In order to clarify histology coding instructions, new rules have been added and histology tables updated. These updates do not require review of already abstracted cases. The December 2020 rules replace the current rules and should be used now. SEER Strongly recommends reading the December 2020 Change Log to understand what changes were made. The updated Solid Tumor Rules may be accessed at: https://seer.cancer.gov/tools/solidtumor/ Reportability Changes for 2021 Radiation Coding Total Dose (1533) Starting 01/01/2021 the following terms are reportable: If doses across phases to a single point of region, code Sum of all phases. ** (see 2019 update below) Early or evolving melanoma in situ, or any other early or If doses are to multiple metastatic sites, code highest evolving melanoma, are reportable. dose site. If doses are to primary site and metastatic site, code dose All GIST tumors are reportable and classified as 8936/3 in from the primary site only. ICD-O-3.2. When you have two different sites, you cannot add the Nearly all thymomas are reportable; the exceptions are doses together to get the total dose. microscopic thymoma or thymoma benign (8580/0), micronodular thymoma with lymphoid stroma (8580/1), Radiation Tips and updates for 2019 and ectopic hamartomatous thymoma (8587/0). -
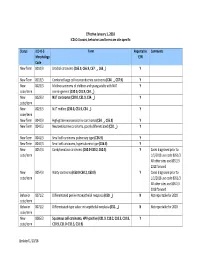
Updated 1/10/18 Effective January 1, 2018 ICD‐O‐3 Codes, Behaviors and Terms Are Site‐Specific Status IC
Effective January 1, 2018 ICD‐O‐3 codes, behaviors and terms are site‐specific Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New Term 8010/3 Urachal carcinoma (C65.9, C66.9, C67. _, C68._) Y New Term 8013/3 Combined large cell neuroendocrine carcinoma (C34. _, C37.9) Y New 8023/3 Midline carcinoma of children and young adults with NUT Y code/term rearrangement (C30.0, C31.9, C34. _) New 8023/3 NUT carcinoma (C30.0, C31.9, C34. _) Y code/term New 8023/3 NUT midline (C30.0, C31.9, C34. _) Y code/term New Term 8041/3 High‐grade neuroendocrine carcinoma (C54. _, C55.9) Y New Term 8041/3 Neuroendocrine carcinoma, poorly differentiated (C50. _) Y New Term 8041/3 Small cell carcinoma pulmonary type (C56.9) Y New Term 8044/3 Small cell carcinoma, hypercalcemic type (C56.9) Y New 8054/3 Condylomatous carcinoma (C60.0‐C60.2, C60.9) Y Cases diagnosed prior to code/term 1/1/2018 use code 8051/3 All other sites use 8051/3 2018 forward New 8054/3 Warty carcinoma (C60.0‐C60.2, C60.9) Y Cases diagnosed prior to code/term 1/1/2018 use code 8051/3 All other sites use 8051/3 2018 forward Behavior 8071/2 Differentiated penile intraepithelial neoplasia (C60. _) N Not reportable for 2018 code/term Behavior 8071/2 Differentiated‐type vulvar intraepithelial neoplasia (C51. _) N Not reportable for 2018 code/term New 8085/3 Squamous cell carcinoma, HPV‐positive (C01.9, C10.2, C10.3, C10.8, Y code/term C10.9, C31.0–C31.3, C31.9) Updated 1/10/18 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New 8086/3 Squamous cell carcinoma, HPV‐negative (C01.9, C10.2, C10.3, Y code/term C10.8, C10.9, C31.0–C31.3, C31.9) New Term 8120/3 Clear cell (glycogen‐rich) urothelial carcinoma (C65.9, C66.9, C67. -

List of Acceptable TCGA Tumor Types*
List of Acceptable TCGA Tumor Types* Acute Myeloid Leukemia Head and Neck Squamous ** Ovarian Carcinoma** Thyroid Papillary Carcinoma** Squamous Cell Carcinoma Serous Cystadenocarcinoma Papillary, Usual Type (Papillary, NOS) Bladder Urothelial Carcinoma Squamous Cell Carcinoma, Spindle Cell Variant Serous carcinoma Papillary, Follicular Variant (99% follicular patterned) Muscle invasive urothelial carcinoma (PT2 or above) Squamous Cell Carcinoma, Basaloid Type Serous adenocarcinoma Papillary, Tall cell Variant (50% tall cell features) Papillary Serous carcinoma Papillary, Other Breast Invasive Carcinoma Hepatocellular Carcinoma Papillary Serous cystoadenocarcinoma Infiltrating Ductal Carcinoma** Hepatocellular Carcinoma, NOS Serous Papillary carcinoma Rare Tumor Studies Infiltrating Lobular Carcinoma Fibrolamellar Hepatocellular Carcinoma Serous Papillary cystoadenocarcinoma Medullary Carcinoma Hepatocholangiocarcinoma (mixed) Serous Papillary adenocarcinoma Adrenocortcal Tumors*** Mucinous Carcinoma Adrenocortical carcinoma - Usual Type Metaplastic Carcinoma Kidney Adenocarcinoma Pancreatic Adenocarcinoma Adrenocortical carcinoma - Oncocytic Type Mixed (with Ductal) Histology*** Clear Cell Renal Cell Carcinoma** Adenocarcinoma, ductal type Adrenocortical carcinoma - Myxoid Type Other Papillary Renal Cell Carcinoma Colloid (mucinous non-cystic) Carcinoma Hepatoid Carcinoma Chromophobe Kidney*** Cervical Cancer Lower Grade Glioma** Medullary Carcinoma Cervical Squamous Cell Carcinoma Astrocytoma, Grade II Signet Ring Cell Carcinoma Mesothelioma -

Labinvest201321.Pdf
190A ANNUAL MEETING ABSTRACTS Sensitivity, specificity, positive/negative predictive value, and the odds ratio of the SCC (0/33) by FISH. No difference of overall survival time was observed between 2 markers were summarized in Table 1. There was no difference in p16 expression amplified and non-amplified groups in EAC patients. between the DG and control group. Conclusions: ALK amplification was present in 7% of EAC cases, but ALK Table 1. Summary of sensitivity, specificity, PPV, NPV, and OR. rearrangement and expression was not present. No ALK amplification, rearrangement Sensitivity (%) Specificity (%) PPV (%) NPV (%) OR and expression were detected in SCC. ALK amplification is not associated with EAC beta-catenin 92.3 76.9 80.0 90.9 40.0 patients’ overall survival. c-met 69.2 61.5 64.3 66.7 3.6 Either 100.0 53.8 68.4 100.0 13.9* Both 61.5 92.3 88.9 70.6 19.2 785 Mitosis-Specific Marker PHH3 Immunostain Is a More Sensitive NPV, negative predictive value; PPV, positive predictive value; OR, odds ratio. *, estimated OR. and Efficient Method To Evaluate the Mitotic Activity in Gastrointestinal Conclusions: The combination of c-met and beta-catenin provides a relatively specific Stromal Tumor (GIST) panel to identify patients with BE who are at increased risk for future development S Zhu, F Lin, ZE Chen. Geisinger Medical Center, Danville, PA. of dysplasia. Background: Mitotic activity is an important prognostic factor in GIST. The accurate identification of mitotic figures on the H&E stained slides could be challenging due to 783 Microfibrillar Associated Protein 5 (MFAP 5): A Marker for processing artifact, degeneration, apoptosis, or lymphocytic infiltration. -

Pulmonary in Trisomy 18 (T18) and Has Been Described Also in Several Disorders Other Than T18
ANNUAL MEETING ABSTRACTS 449A 1867 Infections in a Children’s Hospital Autopsy Population abstract, is useful and may serve as a guideline to define CH, with linear regression +20% J Springer, R Craver. Louisiana State University Health Science Center, New Orleans, of T18 cases and/or linear regression -20% of control cases being the borderline for CH. LA. Background: Despite advances in antimicrobial therapy, infections/inflammatory 1869 Expression of Disialoganglioside GD2 in Neuroblastomas of lesions may frequently cause or contribute to death in children. Patients Treated with Immunotherapy Design: We retrospectively reviewed all autopsies performed at a children’s hospital T Terzic, P Teira, S Cournoyer, M Peuchmaur, H Sartelet. Centre Hospitalier from 1986-2009 and categorized infectious complications as 1)underlying cause Universitaire Sainte-Justine, Montreal, QC, Canada; Hopital Universitaire Robert- of death,2) mechanism of death complicating another underlying cause of death,3) Debre, Paris, France. contributing to death 4) agonal or infections immediately before death 5) incidental. Background: Neuroblastoma, a malignant neoplasm of the sympathetic nervous Infectious complications were then separated into 3-8 year groups to identify trends system, is one of the most aggressive pediatric cancers with a tendency for widespread over the years. dissemination, relapse and a poor long term survival, despite intensive multimodal Results: There were 1369 deaths over 24 years, 608 (44%) underwent autopsy at treatments. Recent use of immunotherapy (a chimeric human-murine monoclonal the hospital, another 122 (8.9%) were coroner’s cases not performed at the hospital. antibody ch14.18 directed against a tumor-associated antigen GD2, a disialoganglioside) There were 691 infectious conditions in 401 children (66%, 1.72 infections/infected to treat minimal residual disease has shown improvement in event-free and overall patient).There were no differences in the percentage of autopsies with infections over survival of high-risk neuroblastomas. -

1 Supplement Supplement Table 1. CD274 Loss, Neutral, and Gain in Various Diagnosis
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Supplement Supplement Table 1. CD274 loss, neutral, and gain in various diagnosis CD274 CD274 CD274 Diagnosis N Loss Neutral Gain (%) (%) (%) lung adenocarcinoma 30396 48.5 36.3 15.1 colon adenocarcinoma (crc) 25618 19.3 55.6 25.0 breast carcinoma (nos) 14679 35.8 44.0 20.1 pancreas ductal adenocarcinoma 11698 56.3 35.2 8.5 prostate acinar adenocarcinoma 9466 28.6 55.0 16.4 lung squamous cell carcinoma (scc) 8852 52.6 29.5 17.9 breast invasive ductal carcinoma (idc) 8620 32.9 42.2 24.8 ovary serous carcinoma 7824 39.4 40.7 19.8 lung non-small cell lung carcinoma (nsclc) 6944 48.3 32.8 19.0 (nos) unknown primary adenocarcinoma 6329 48.2 38.0 13.8 rectum adenocarcinoma (crc) 5186 20.5 53.9 25.6 brain glioblastoma (gbm) 4787 36.1 54.6 9.3 liver cholangiocarcinoma 4653 48.0 39.8 12.2 bladder urothelial (transitional cell) 4443 42.0 39.6 18.4 carcinoma esophagus adenocarcinoma 4138 50.0 38.0 12.0 stomach adenocarcinoma (nos) 3979 40.6 45.0 14.4 head and neck squamous cell carcinoma 3511 31.0 43.3 25.7 (hnscc) skin melanoma 3501 55.4 36.6 8.1 unknown primary carcinoma (cup) (nos) 3432 41.1 40.2 18.7 uterus endometrial adenocarcinoma (nos) 3331 24.7 60.8 14.5 unknown primary melanoma 3038 51.4 37.7 11.0 ovary epithelial carcinoma 2564 37.7 41.8 20.5 lung small cell undifferentiated carcinoma 2493 21.4 51.6 27.0 pancreatobiliary carcinoma 2178 55.1 35.0 10.0 uterus endometrial adenocarcinoma 2113 16.6 71.7 11.7 endometrioid ovary high grade serous carcinoma 2025 41.1 37.8 21.1 uterus endometrial adenocarcinoma 1971 31.7 46.8 21.6 papillary serous kidney renal cell carcinoma 1675 48.2 40.1 11.7 gallbladder adenocarcinoma 1486 55.5 34.4 10.2 pancreas carcinoma (nos) 1481 55.1 34.4 10.5 gastroesophageal junction adenocarcinoma 1477 48.1 38.9 12.9 unknown primary squamous cell carcinoma 1414 39.7 36.8 23.6 (scc) 1 Huang RS.P, et al. -

Comparison of Tumour Markers in Malignant Mesothelioma and Pulmonary Adenocarcinoma
Thorax: first published as 10.1136/thx.40.2.91 on 1 February 1985. Downloaded from Thorax 1985;40:91-95 Comparison of tumour markers in malignant mesothelioma and pulmonary adenocarcinoma AR GIBBS, R HARACH, JC WAGNER, B JASANI From the Pathology Department, Welsh National School, Cardiff, and the Medical Research Council Pneumoconiosis Unit, Llandough Hospital, Penarth, Glamorgan ABSTRACr Immunohistological methods were used to investigate the presence of carcinoem- bryonic antigen, ,31 pregnancy specific glycoprotein, /8 subunit of human chorionic gonado- trophin, human placental lactogen, calcitonin, and keratin in formalin fixed tissue from 29 malig- nant mesotheliomas and 27 pulmonary adenocarcinomas. Malignant mesotheliomas were nega- tive for tumour markers except for the ,8 subunit of human chorionic gonadotrophin and keratin, one and 13 cases respectively being positive for these. Pulmonary adenocarcinomas, however, were frequently positive for tumour markers-namely, carcinoembryonic antigen (24), ,3B preg- nancy specific glycoprotein (23), ,8 subunit of human chorionic gonadotrophin (8), human placen- tal lactogen (2), calcitonin (3), and keratin (12). The presence of carcinoembryonic antigen and ,/3 pregnancy specific glycoprotein within an intrathoracic tumour is strong evidence against its being of mesothelial origin. copyright. The accurate diagnosis of malignant tumours is an needs to be recognised well in advance, so that the essential part of the management of patients, but for appropriate fixation and embedding techniques -

TNM Classification of Malignant Tumours
Table of Contents Cover Title Page Preface References Acknowledgments Organizations Associated with the TNM System Members of UICC Committees Associated with the TNM System Section Editors Introduction References Head and Neck Tumours Lip and Oral Cavity Pharynx References Larynx Nasal Cavity and Paranasal Sinuses Unknown Primary – Cervical Nodes Malignant Melanoma of Upper Aerodigestive Tract Major Salivary Glands Thyroid Gland Digestive System Tumours Oesophagus Stomach Reference Small Intestine Appendix Colon and Rectum Anal Canal and Perianal Skin Liver Intrahepatic Bile Ducts Gallbladder Perihilar Bile Ducts Distal Extrahepatic Bile Duct Ampulla of Vater Pancreas Well Differentiated Neuroendocrine Tumours of the Gastrointestinal Tract Lung, Pleural, and Thymic Tumours Lung References Pleural Mesothelioma Thymic Tumours References Tumours of Bone and Soft Tissues Bone Soft Tissues Gastrointestinal Stromal Tumour (GIST) Skin Tumours Carcinoma of Skin (excluding eyelid, head and neck, perianal, vulva, and penis) Skin Carcinoma of the Head and Neck Carcinoma of Skin of the Eyelid Malignant Melanoma of Skin Merkel Cell Carcinoma of Skin Breast Tumours Reference Gynaecological Tumours Reference Vulva Vagina Cervix Uteri Uterus – Endometrium Reference Uterine Sarcomas References Ovarian, Fallopian Tube, and Primary Peritoneal Carcinoma Reference Gestational Trophoblastic Neoplasms Reference Urological Tumours Penis Prostate References Testis Kidney Renal Pelvis and Ureter Urinary Bladder Urethra Adrenal Cortex (ICD O 3 C74.0) Ophthalmic