Pulmonary in Trisomy 18 (T18) and Has Been Described Also in Several Disorders Other Than T18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Influence of Iatrogenic Atrial Septal Defect On
Bioscience Reports (2020) 40 BSR20193128 https://doi.org/10.1042/BSR20193128 Research Article The influence of iatrogenic atrial septal defect on the prognosis of patients with atrial fibrillation between cryoablation and radiofrequency ablation Ying Yang, Jinglan Wu, Lixia Yao, Yue Liu, Chenfeng Zhang, Ling You, Jing Yang and Ruiqin Xie Downloaded from http://portlandpress.com/bioscirep/article-pdf/40/2/BSR20193128/867399/bsr-2019-3128.pdf by guest on 02 October 2021 Department of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, China Correspondence: Ruiqin Xie ([email protected]) Objective: The present study was to compare the incidence of septal defect (SD) in patients with atrial fibrillation (AF) who received radiofrequency ablation or cryoablation. Methods: A total of 293 AF patients were performed with radiofrequency ablation and cryoablation. Cardiac ultrasonography was performed to calculate left atrial diameter (LAD), left atrial ejection fraction (LAEF%), strain rate (SR), left ventricular systolic (SRs), left ven- tricular diastolic (SRe), and left atrial systole (SRa) before surgery, 3 months and 1 year after surgery. The patients were followed up to observe statin and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) medication, AF recurrence, 6-min walk test, stroke, any symptoms caused by arrhythmia, and re-hospitalization. Results: The lev- els of LAD and SD were higher, while SRe and SRa were lower in the cryoablation group in the comparison with the radiofrequency ablation group after surgery (P<0.05). LAEF was lower in the cryoablation group than the radiofrequency ablation group after 3 months (P<0.05). After 1-year follow-up, no right-to-left shunt occurred in all patients with SD. -

Table of Contents
Version 4.9.2019 Table of Contents Abdominal Transplantation 1 Cardiothoracic Transplantation & Circulatory Support 31 Cardiothoracic Surgery 50 Congenital Heart Surgery 84 General Surgery 95 General Thoracic Surgery 145 Pediatric Surgery 152 Plastic Surgery 199 Surgical Oncology 223 Vascular Surgery and Endovascular Therapy 258 Office of Surgical Research 282 ABDOMINAL TRANSPLANTATION The division is committed to clinical and basic research, in part funded through NIH grants, in areas such as adult and pediatric solid organ transplantation, liver disease, kidney disease, immunogenetics, bone marrow transplant and chronic hepatitis C. Led by Dr. John M. Vierling, professor and chief of hepatology, the Baylor St. Luke’s Advanced Liver Therapies Research Center gives patients access to clinical trials offering the latest therapies. Co-directed by Drs. Peter Jindra and Matt Cusick, the division- run Immune Evaluation Laboratory continues to expand its research activities while remaining the largest program of its kind in the Texas Medical Center John A Goss, M.D., F.A.C.S. Professor of Surgery and Chief, Division of Abdominal Transplantation Baylor College of Medicine JLH Foundation Chair in Transplant Surgery - Texas Children's Hospital Director of Liver Transplantation - Baylor St. Luke's Medical Center Director of Liver Transplantation - Texas Children's Hospital Director of Liver Transplantation - Michael E. DeBakey Veterans Affairs Medical Center Keywords • Adult and pediatric liver transplantation • Biliary resection/reconstruction • Bile duct tumor • Bile duct injury • Cirrhosis • Hepatobiliary surgery • Liver disease • Liver resection • Liver tumors • Portal hypertension • Portosystemic shunts • Radio frequency ablation • Sugiura procedure • Surgical management of liver tumors Research Interests Dr. Goss' primary research interests revolve around the genomic alterations that occur with hepatocellular carcinoma. -

Advancement in Diagnostic Imaging of Thymic Tumors
cancers Commentary Advancement in Diagnostic Imaging of Thymic Tumors Francesco Gentili 1,*, Ilaria Monteleone 2, Francesco Giuseppe Mazzei 1, Luca Luzzi 3, Davide Del Roscio 2, Susanna Guerrini 1 , Luca Volterrani 2 and Maria Antonietta Mazzei 2 1 Unit of Diagnostic Imaging, Department of Radiological Sciences, Azienda Ospedaliero-Universitaria Senese, 53100 Siena, Italy; [email protected] (F.G.M.); [email protected] (S.G.) 2 Unit of Diagnostic Imaging, Department of Medical, Surgical and Neuro Sciences and of Radiological Sciences, University of Siena, Azienda Ospedaliero-Universitaria Senese, 53100 Siena, Italy; [email protected] (I.M.); [email protected] (D.D.R.); [email protected] (L.V.); [email protected] (M.A.M.) 3 Thoracic Surgery Unit, Department of Medical, Surgical and Neuro Sciences, University of Siena, Azienda Ospedaliero-Universitaria Senese, 53100 Siena, Italy; [email protected] * Correspondence: [email protected] Simple Summary: Diagnostic imaging is pivotal for the diagnosis and staging of thymic tumors. It is important to distinguish thymoma and other tumor histotypes amenable to surgery from lymphoma. Furthermore, in cases of thymoma, it is necessary to differentiate between early and advanced disease before surgery since patients with locally advanced tumors require neoadjuvant chemotherapy for improving survival. This review aims to provide to radiologists a full spectrum of findings of thymic neoplasms using traditional and innovative imaging modalities. Abstract: Thymic tumors are rare neoplasms even if they are the most common primary neoplasm of the anterior mediastinum. In the era of advanced imaging modalities, such as functional MRI, dual- Citation: Gentili, F.; Monteleone, I.; Mazzei, F.G.; Luzzi, L.; Del Roscio, D.; energy CT, perfusion CT and radiomics, it is possible to improve characterization of thymic epithelial Guerrini, S.; Volterrani, L.; Mazzei, tumors and other mediastinal tumors, assessment of tumor invasion into adjacent structures and M.A. -

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

Copy Number Aberrations of Genes Regulating Normal Thymus Development in Thymic Epithelial Tumors
Published OnlineFirst February 26, 2013; DOI: 10.1158/1078-0432.CCR-12-3260 Clinical Cancer Human Cancer Biology Research Copy Number Aberrations of Genes Regulating Normal Thymus Development in Thymic Epithelial Tumors Iacopo Petrini1, Yisong Wang1, Paolo A. Zucali3, Hye Seung Lee1,4, Trung Pham1, Donna Voeller1, Paul S. Meltzer2, and Giuseppe Giaccone1 Abstract Purposes: To determine whether the deregulation of genes relevant for normal thymus development can contribute to the biology of thymic epithelial tumors (TET). Experimental Design: Using array comparative genomic hybridization, we evaluated the copy number aberrations of genes regulating thymus development. The expression of genes most commonly involved in copy number aberrations was evaluated by immunohistochemistry and correlated with patients’ outcome. Correlation between FOXC1 copy number loss and gene expression was determined in a confirmation cohort. Cell lines were used to test the role of FOXC1 in tumors. Results: Among 31 thymus development-related genes, PBX1 copy number gain and FOXC1 copy number loss were presented in 43.0% and 39.5% of the tumors, respectively. Immunohistochemistry on a series of 132 TETs, including those evaluated by comparative genomic hybridization, revealed a correlation between protein expression and copy number status only for FOXC1 but not for PBX1. Patients with FOXC1- negative tumors had a shorter time to progression and a trend for a shorter disease-related survival. The correlation between FOXC1 copy number loss and mRNA expression was confirmed in a separate cohort of 27 TETs. Ectopic FOXC1 expression attenuated anchorage-independent cell growth and cell migration in vitro. Conclusion: Our data support a tumor suppressor role of FOXC1 in TETs. -

Variants of Acinar Adenocarcinoma of the Prostate Mimicking Benign Conditions Peter a Humphrey
Modern Pathology (2018) 31, S64–S70 S64 © 2018 USCAP, Inc All rights reserved 0893-3952/18 $32.00 Variants of acinar adenocarcinoma of the prostate mimicking benign conditions Peter A Humphrey Department of Pathology, Yale School of Medicine, New Haven, CT, USA Histological variants of acinar adenocarcinoma of the prostate may be of significance due to difficulty in diagnosis or due to differences in prognosis compared to usual acinar adenocarcinoma. The 2016 World Health Organization classification of acinar adenocarcinoma includes four variants that are deceptively benign in histological appearance, such that a misdiagnosis of a benign condition may be made. These four variants are atrophic pattern adenocarcinoma, pseudohyperplastic adenocarcinoma, microcystic adenocarcinoma, and foamy gland adenocarcinoma. They differ from usual small acinar adenocarcinoma in architectural glandular structure and/or cytoplasmic and nuclear alterations. The variants are often admixed, in variable proportions, with usual small acinar adenocarcinoma that is often Gleason pattern 3 but may be high-grade pattern 4 in a minority of cases. Atrophic pattern adenocarcinoma can be identified in a sporadic setting or after radiation or hormonal therapy. This variant is characterized by cytoplasmic volume loss and can resemble benign glandular atrophy, an extremely common benign process in the prostate. The glands of pseudohyperplastic adenocarcinoma simulate usual epithelial hyperplasia, with gland complexity that is not typical of small acinar adenocarcinoma. These complex growth configurations include papillary infoldings, luminal undulations, and branching. Microcystic adenocarcinoma is characterized by cystic dilation of prostatic glands to a size that is much more commonly observed in cystic change in benign prostatic glands. Finally, the cells in foamy gland adenocarcinoma display cytoplasmic vacuolization and nuclear pyknosis, features that can found in benign glands and macrophages. -

Supplementary Table 1) Immunohistochemical Protocol and Antibody Information Antibody Company Clone/# Clonality Dilution Incubat
H. Reis et al: Differential proteomic and tissue expression analyses identify valuable diagnostic biomarkers of hepatocellular differentiation and hepatoid adenocarcinomas Suppl ementary Table 1) Immunohistochemical protocol and antibody information Antibody Company Clone/# Clonality Dilution Incubation Antigen retrieval Detection ABAT Abcam EPR4433 mono 1/3000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP ACAA2 Abcam EPR6733 mono 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP ACADM Abcam EPR3708 mono 1/3000 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP ADH1B Abcam 4F12 mono 1/12.000 30 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP Arginase1 Abcam EPR6672(B) mono 1/1000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP BHMT Abcam EPR6782 mono 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP FABP1 Acris AIV\09011PU-S mono 1/15.000 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HAOX1 Acris AP18044PU-N poly 1/100 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HepPar1 Dako OCH1E5 mono 1/800 30 min, RT pH 9.0, WB, 95°C, 20 min. Zytomed Polymer HRP HMGCS2 Abcam Ab104807 poly 1/50 60 min, RT pH 9.0, WB, 95°C, 20 min Zytomed Polymer HRP H. Reis et al: Differential proteomic and tissue expression analyses identify valuable diagnostic biomarkers of hepatocellular differentiation and hepatoid adenocarcinomas Supplementary Table 2) Composition of the non-liver tumor TMA Diagnosis n % ADC 11 2.9 ADC Adenocarcinoma of the lung Carc. -

1 Effective January 1, 2018 ICD‐O‐3 Codes, Behaviors and Terms Are Site‐Specific Alpha Order Last Updat
Effective January 1, 2018 ICD‐O‐3 codes, behaviors and terms are site‐specific Alpha Order Last updated 8/22/18 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New Term 8551/3 Acinar adenocarcinoma (C34. _) Y Lung primaries diagnosed prior to 1/1/2018 use code 8550/3 For prostate (all years) see 8140/3 New Term 8140/3 Acinar adenocarcinoma (C61.9 ONLY) Y For prostate only, do not use 8550/3 New Term 8572/3 Acinar adenocarcinoma, sarcomatoid (C61.9) Y New Term 8550/3 Acinar cell carcinoma Y Excludes C61.9‐ see 8140/3 New Term 8316/3 Acquired cystic disease‐associated renal cell carcinoma (RCC) Y (C64.9) New 8158/1 ACTH‐producing tumor N code/term New Term 8574/3 Adenocarcinoma admixed with neuroendocrine carcinoma (C53. _) Y Behavior 8253/2 Adenocarcinoma in situ, mucinous (C34. _) Y Important note: lung Code/term primaries ONLY: For cases diagnosed 1/1/2018 forward do not use code 8480 (mucinous adenocarcinoma) for in‐ situ adenocarcinoma, mucinous or invasive mucinous adenocarcinoma. 1 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code Behavior 8250/2 Adenocarcinoma in situ, non‐mucinous (C34. _) Y code/term New Term 9110/3 Adenocarcinoma of rete ovarii (C56.9) Y New 8163/3 Adenocarcinoma, pancreatobiliary‐type (C24.1) Y Cases diagnosed prior to code/term 1/1/2018 use code 8255/3 Behavior 8983/3 Adenomyoepithelioma with carcinoma (C50. _) Y Code/term New Term 8620/3 Adult granulosa cell tumor (C56.9 ONLY) N Not reportable for 2018 cases New Term 9401/3 Anaplastic astrocytoma, IDH‐mutant (C71. -

Einstein Healthcare Network Cancer Center Active
EINSTEIN HEALTHCARE NETWORK CANCER CENTER ACTIVE CLINICAL TRIALS 1 TABLE OF CONTENTS PAGE Studies by Organ/System 3 Brain 4 - 6 Breast – Neoadjuvant/Adjuvant 6 Breast – Advanced/Metastatic 7-8 Gastrointestinal – Colorectal – Adjuvant 9 Gastrointestinal –Advanced, Metastatic 10 Gastrointestinal –Hepatocellular 11 Gastrointestinal –Pancreatic 12-13 Genitourinary – Prostate 14 Gynecological 15-16 Head and Neck 17 Myeloma 18-20 Lung – NSCLC 21-22 Lung – SCLC/Thymoma, Thymic Carcinoma/Mesothelioma 23-24 Supportive care 25 Multiple cancer diagnoses 26 Trials pending activation 27 ECOG Path. Coordinating Ctr Information “NEW” 10/24/2014 2 BRAIN PROTOCOL CONTACTS Radiation Oncology Investigator – Kenneth Zeitzer, MD 215-456-6280 Coordinator – Jeff Mealey, RN 215-456-6316 No active studies at this time. 3 BREAST PROTOCOL CONTACTS MEDICAL ONCOLOGY Investigator – Mark S. Morginstin, DO 215-456-3880 Coordinator – Joann R. Ackler, RN, OCN, CCRP 215-456-8295 RADIATION ONCOLOGY Investigator – Angelica T. Montesano, MD 215-456-6280 Coordinator – Jeff Mealy, RN 215-456-6316 STUDY# TITLE and ELIGIBILITY CRITERIA THERAPY ADJUVANT NRG BR-003/ A Randomized Phase III Trial of Adjuvant Therapy Arm 1: CIRB-005 Comparing Doxorubicin Plus Cyclophosphamide Followed by Doxorubicin 60 mg/M2 IV Weekly Paclitaxel with or without Carboplatin for Node- Cyclophosphamide 600 mg/m2 IV # Pts: _____ Positive or High-Risk Node Negative Invasive Breast Cancer Q 2 Weeks X 4 cycles Eligibility: Unilateral breast IDC: pT1-3; pN0 – pN3b, Followed by: underwent mastectomy or clear margins on lumpectomy/re- Paclitaxel 80 mg/m2 IV weekly x 12 doses excision; ER, PR, & HER2 negative (please see pgs. 14-15 of protocol for specifics). -

December 2020 E-Tips Solid Tumor Rules
New Jersey State Cancer Registry December 2020 E-Tips Cancer Epidemiology Services http://www.nj.gov/health/ces (609) 633-0500 Solid Tumor Rules: December 2020 Update ICD-0-3.2 changes have also been added to applicable site modules. Most changes are minor: terminology, additional definitions, new notes and examples. In order to clarify histology coding instructions, new rules have been added and histology tables updated. These updates do not require review of already abstracted cases. The December 2020 rules replace the current rules and should be used now. SEER Strongly recommends reading the December 2020 Change Log to understand what changes were made. The updated Solid Tumor Rules may be accessed at: https://seer.cancer.gov/tools/solidtumor/ Reportability Changes for 2021 Radiation Coding Total Dose (1533) Starting 01/01/2021 the following terms are reportable: If doses across phases to a single point of region, code Sum of all phases. ** (see 2019 update below) Early or evolving melanoma in situ, or any other early or If doses are to multiple metastatic sites, code highest evolving melanoma, are reportable. dose site. If doses are to primary site and metastatic site, code dose All GIST tumors are reportable and classified as 8936/3 in from the primary site only. ICD-O-3.2. When you have two different sites, you cannot add the Nearly all thymomas are reportable; the exceptions are doses together to get the total dose. microscopic thymoma or thymoma benign (8580/0), micronodular thymoma with lymphoid stroma (8580/1), Radiation Tips and updates for 2019 and ectopic hamartomatous thymoma (8587/0). -
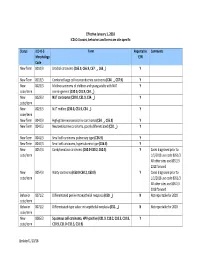
Updated 1/10/18 Effective January 1, 2018 ICD‐O‐3 Codes, Behaviors and Terms Are Site‐Specific Status IC
Effective January 1, 2018 ICD‐O‐3 codes, behaviors and terms are site‐specific Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New Term 8010/3 Urachal carcinoma (C65.9, C66.9, C67. _, C68._) Y New Term 8013/3 Combined large cell neuroendocrine carcinoma (C34. _, C37.9) Y New 8023/3 Midline carcinoma of children and young adults with NUT Y code/term rearrangement (C30.0, C31.9, C34. _) New 8023/3 NUT carcinoma (C30.0, C31.9, C34. _) Y code/term New 8023/3 NUT midline (C30.0, C31.9, C34. _) Y code/term New Term 8041/3 High‐grade neuroendocrine carcinoma (C54. _, C55.9) Y New Term 8041/3 Neuroendocrine carcinoma, poorly differentiated (C50. _) Y New Term 8041/3 Small cell carcinoma pulmonary type (C56.9) Y New Term 8044/3 Small cell carcinoma, hypercalcemic type (C56.9) Y New 8054/3 Condylomatous carcinoma (C60.0‐C60.2, C60.9) Y Cases diagnosed prior to code/term 1/1/2018 use code 8051/3 All other sites use 8051/3 2018 forward New 8054/3 Warty carcinoma (C60.0‐C60.2, C60.9) Y Cases diagnosed prior to code/term 1/1/2018 use code 8051/3 All other sites use 8051/3 2018 forward Behavior 8071/2 Differentiated penile intraepithelial neoplasia (C60. _) N Not reportable for 2018 code/term Behavior 8071/2 Differentiated‐type vulvar intraepithelial neoplasia (C51. _) N Not reportable for 2018 code/term New 8085/3 Squamous cell carcinoma, HPV‐positive (C01.9, C10.2, C10.3, C10.8, Y code/term C10.9, C31.0–C31.3, C31.9) Updated 1/10/18 Status ICD‐O‐3 Term Reportable Comments Morphology Y/N Code New 8086/3 Squamous cell carcinoma, HPV‐negative (C01.9, C10.2, C10.3, Y code/term C10.8, C10.9, C31.0–C31.3, C31.9) New Term 8120/3 Clear cell (glycogen‐rich) urothelial carcinoma (C65.9, C66.9, C67. -

List of Acceptable TCGA Tumor Types*
List of Acceptable TCGA Tumor Types* Acute Myeloid Leukemia Head and Neck Squamous ** Ovarian Carcinoma** Thyroid Papillary Carcinoma** Squamous Cell Carcinoma Serous Cystadenocarcinoma Papillary, Usual Type (Papillary, NOS) Bladder Urothelial Carcinoma Squamous Cell Carcinoma, Spindle Cell Variant Serous carcinoma Papillary, Follicular Variant (99% follicular patterned) Muscle invasive urothelial carcinoma (PT2 or above) Squamous Cell Carcinoma, Basaloid Type Serous adenocarcinoma Papillary, Tall cell Variant (50% tall cell features) Papillary Serous carcinoma Papillary, Other Breast Invasive Carcinoma Hepatocellular Carcinoma Papillary Serous cystoadenocarcinoma Infiltrating Ductal Carcinoma** Hepatocellular Carcinoma, NOS Serous Papillary carcinoma Rare Tumor Studies Infiltrating Lobular Carcinoma Fibrolamellar Hepatocellular Carcinoma Serous Papillary cystoadenocarcinoma Medullary Carcinoma Hepatocholangiocarcinoma (mixed) Serous Papillary adenocarcinoma Adrenocortcal Tumors*** Mucinous Carcinoma Adrenocortical carcinoma - Usual Type Metaplastic Carcinoma Kidney Adenocarcinoma Pancreatic Adenocarcinoma Adrenocortical carcinoma - Oncocytic Type Mixed (with Ductal) Histology*** Clear Cell Renal Cell Carcinoma** Adenocarcinoma, ductal type Adrenocortical carcinoma - Myxoid Type Other Papillary Renal Cell Carcinoma Colloid (mucinous non-cystic) Carcinoma Hepatoid Carcinoma Chromophobe Kidney*** Cervical Cancer Lower Grade Glioma** Medullary Carcinoma Cervical Squamous Cell Carcinoma Astrocytoma, Grade II Signet Ring Cell Carcinoma Mesothelioma