New Developments in Imaging for Sentinel Lymph Node Biopsy in Early-Stage Oral Cavity Squamous Cell Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Follicular Lymphoma
Follicular Lymphoma What is follicular lymphoma? Let us explain it to you. www.anticancerfund.org www.esmo.org ESMO/ACF Patient Guide Series based on the ESMO Clinical Practice Guidelines FOLLICULAR LYMPHOMA: A GUIDE FOR PATIENTS PATIENT INFORMATION BASED ON ESMO CLINICAL PRACTICE GUIDELINES This guide for patients has been prepared by the Anticancer Fund as a service to patients, to help patients and their relatives better understand the nature of follicular lymphoma and appreciate the best treatment choices available according to the subtype of follicular lymphoma. We recommend that patients ask their doctors about what tests or types of treatments are needed for their type and stage of disease. The medical information described in this document is based on the clinical practice guidelines of the European Society for Medical Oncology (ESMO) for the management of newly diagnosed and relapsed follicular lymphoma. This guide for patients has been produced in collaboration with ESMO and is disseminated with the permission of ESMO. It has been written by a medical doctor and reviewed by two oncologists from ESMO including the lead author of the clinical practice guidelines for professionals, as well as two oncology nurses from the European Oncology Nursing Society (EONS). It has also been reviewed by patient representatives from ESMO’s Cancer Patient Working Group. More information about the Anticancer Fund: www.anticancerfund.org More information about the European Society for Medical Oncology: www.esmo.org For words marked with an asterisk, a definition is provided at the end of the document. Follicular Lymphoma: a guide for patients - Information based on ESMO Clinical Practice Guidelines – v.2014.1 Page 1 This document is provided by the Anticancer Fund with the permission of ESMO. -

TNM Staging of Head and Neck Cancer and Neck Dissection Classification
QUICK REFERENCE GUIDE TO TNM Staging of Head and Neck Cancer and Neck Dissection Classification Fourth Edition © 2014 All materials in this eBook are copyrighted by the American Academy of Otolaryngology— Head and Neck Surgery Foundation, 1650 Diagonal Road, Alexandria, VA 22314-2857, and the American Head and Neck Society, 11300 W. Olympic Blvd., Suite 600, Los Angeles CA 90064, and are strictly prohibited to be used for any purpose without prior written authorization from the American Academy of Otolaryngology— Head and Neck Surgery Foundation and the American Head and Neck Society. All rights reserved. For more information, visit our website at www.entnet.org , or www.ahns.org. eBook Format: Fourth Edition, 2014 ISBN: 978-0-615-98874-0 Suggested citation: Deschler DG, Moore MG, Smith RV, eds. Quick Reference Guide to TNM Staging of Head and Neck Cancer and Neck Dissection Classification, 4th ed. Alexandria, VA: American Academy of Otolaryngology–Head and Neck Surgery Foundation, 2014. Quick Reference Guide to TNM Staging of Head and Neck Cancer and Neck Dissection Classification Copublished by American Academy of Otolaryngology—Head and Neck Surgery American Head and Neck Society Edited by Daniel G. Deschler, MD Michael G. Moore, MD Richard V. Smith, MD Table of Contents Preface ................................................................................................................................iv Acknowledgments ...........................................................................................................v I. -

December 2020 E-Tips Solid Tumor Rules
New Jersey State Cancer Registry December 2020 E-Tips Cancer Epidemiology Services http://www.nj.gov/health/ces (609) 633-0500 Solid Tumor Rules: December 2020 Update ICD-0-3.2 changes have also been added to applicable site modules. Most changes are minor: terminology, additional definitions, new notes and examples. In order to clarify histology coding instructions, new rules have been added and histology tables updated. These updates do not require review of already abstracted cases. The December 2020 rules replace the current rules and should be used now. SEER Strongly recommends reading the December 2020 Change Log to understand what changes were made. The updated Solid Tumor Rules may be accessed at: https://seer.cancer.gov/tools/solidtumor/ Reportability Changes for 2021 Radiation Coding Total Dose (1533) Starting 01/01/2021 the following terms are reportable: If doses across phases to a single point of region, code Sum of all phases. ** (see 2019 update below) Early or evolving melanoma in situ, or any other early or If doses are to multiple metastatic sites, code highest evolving melanoma, are reportable. dose site. If doses are to primary site and metastatic site, code dose All GIST tumors are reportable and classified as 8936/3 in from the primary site only. ICD-O-3.2. When you have two different sites, you cannot add the Nearly all thymomas are reportable; the exceptions are doses together to get the total dose. microscopic thymoma or thymoma benign (8580/0), micronodular thymoma with lymphoid stroma (8580/1), Radiation Tips and updates for 2019 and ectopic hamartomatous thymoma (8587/0). -

Neck Dissection & Ajcc 8Th Edition
NECK DISSECTION & NODAL STAGING CHERIE-ANN NATHAN, MD, FACS JACK W. POU ENDOWED PROF. & CHAIRMAN DIRECTOR HEAD & NECK Feist-Weiller Cancer Ctr, DEPT. OF OTOLARYNGOLOGY/HNS, LSU HEALTH-SHV HISTORY • 1906 : George Crile described the classic radical neck dissection (RND) • 1933 and 1941 : Blair and Martin popularized the RND • 1975 : Bocca established oncologic safety of the FND compared to the RND • 1989, 1991, and 1994: Medina, Robbins, and Byers respectively proposed classifications of neck dissections Proposed Classification, Ferlito et al, AAO-HNS Revised Classification, 2008 2011 ND (I–V, SCM, IJV, CN XI) Radical neck dissection ND (I–V, SCM, IJV, CN XI, Extended neck dissection and CN XII) with removal of the hypoglossal nerve ND (I–V, SCM, IJV) Modified radical neck dissection with preservation of the spinal accessory nerve ND (II–IV) Selective neck dissection (II–IV) ND (II–IV, VI) Selective neck dissection (II–IV, VI) ND (II–IV, SCM) NA ND (I–III) Selective neck dissection (I–III) RELEVANT ANATOMY from www.entnet.org/academyU Definition of cN0 neck • Absence of palpable adenopathy on physical examination • Absence of visual adenopathy on CT or MRI or PET Risk of micrometastases in the N0 neck Specific cancers arising in selected mucosal sites have a low risk of metastases: T1 glottic carcinoma T1-2 lip cancers Thin (<4 mm) oral cavity cancers Most carcinomas of the UADT have a minimum of 15% risk of metastases Treatment options for the N0 neck • Observation • Neck dissection • Radiation therapy • Sentinel node dissection ALGORITHM -

Head and Neck Specimens
Head and Neck Specimens DEFINITIONS AND GENERAL COMMENTS: All specimens, even of the same type, are unique, and this is particularly true for Head and Neck specimens. Thus, while this outline is meant to provide a guide to grossing the common head and neck specimens at UAB, it is not all inclusive and will not capture every scenario. Thus, careful assessment of each specimen with some modifications of what follows below may be needed on a case by case basis. When in doubt always consult with a PA, Chief/Senior Resident and/or the Head and Neck Pathologist on service. Specimen-derived margin: A margin taken directly from the main specimen-either a shave or radial. Tumor bed margin: A piece of tissue taken from the operative bed after the main specimen has been resected. This entire piece of tissue may represent the margin, or it could also be specifically oriented-check specimen label/requisition for any further orientation. Margin status as determined from specimen-derived margins has been shown to better predict local recurrence as compared to tumor bed margins (Surgical Pathology Clinics. 2017; 10: 1-14). At UAB, both methods are employed. Note to grosser: However, even if a surgeon submits tumor bed margins separately, the grosser must still sample the specimen margins. Figure 1: Shave vs radial (perpendicular) margin: Figure adapted from Surgical Pathology Clinics. 2017; 10: 1-14): Red lines: radial section (perpendicular) of margin Blue line: Shave of margin Comparison of shave and radial margins (Table 1 from Chiosea SI. Intraoperative Margin Assessment in Early Oral Squamous Cell Carcinoma. -

About Mastectomy and Sentinel Lymph Node Biopsy
All About Mastectomy and Sentinel Lymph Node Biopsy One of the most important goals of Moffitt Cancer Center is to provide you with quality patient care through education, research and patient care. The following information has been developed to help you understand the mastectomy and sentinel lymph node biopsy procedures for which you have been scheduled. Members of your health care team will review this information with you and answer any questions that you may have. Definitions: Mastectomy – Removal of the entire breast but not the muscles underneath. Lymph Nodes - Small bean shaped glands found in the armpit. They remove waste and fluids from the arm and breast and help fight infection. They are also call lymph glands. Sentinel Lymph Node - The first place breast cancer may metastasize (or spread) is to lymph nodes in the axilla (underarm area) on the side of the body where the cancer is. The first lymph node(s) are referred to as the sentinel lymph node(s) or the “gate keepers”. These nodes are first biopsied to determine if the cancer has spread. If cancer cells are not found in the sentinel lymph node(s) that were removed, it will not be necessary to remove the remaining lymph nodes in the arm pit. Sentinel Lymph Node Mapping and Biopsy - This procedure is a two step process. Performing the sentinel lymph node biopsy requires a small incision in your axilla. In the first step, the doctor injects a radioactive substance and/ or blue dye in the area around the tumor. Lymphatic channels (like blood vessels) carry these materials to the sentinel lymph node. -

Consensus Guideline on the Management of the Axilla in Patients with Invasive/In-Situ Breast Cancer
- Official Statement - Consensus Guideline on the Management of the Axilla in Patients With Invasive/In-Situ Breast Cancer Purpose To outline the management of the axilla for patients with invasive and in-situ breast cancer. Associated ASBrS Guidelines or Quality Measures 1. Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients – Revised November 25, 2014 2. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients – Approved November 25, 2014 3. Quality Measure: Sentinel Lymph Node Biopsy for Invasive Breast Cancer – Approved November 4, 2010 4. Prior Position Statement: Management of the Axilla in Patients With Invasive Breast Cancer – Approved August 31, 2011 Methods A literature review inclusive of recent randomized controlled trials evaluating the use of sentinel lymph node surgery and axillary lymph node dissection for invasive and in-situ breast cancer as well as the pathologic review of sentinel lymph nodes and indications for axillary radiation was performed. This is not a complete systematic review but rather, a comprehensive review of recent relevant literature. A focused review of non-randomized controlled trials was then performed to develop consensus guidance on management of the axilla in scenarios where randomized controlled trials data is lacking. The ASBrS Research Committee developed a consensus document, which was reviewed and approved by the ASBrS Board of Directors. Summary of Data Reviewed Recommendations Based on Randomized Controlled -

Application of Preoperative Computed Tomographic Lymphography For
Wen et al. BMC Surg (2021) 21:187 https://doi.org/10.1186/s12893-021-01190-7 RESEARCH ARTICLE Open Access Application of preoperative computed tomographic lymphography for precise sentinel lymph node biopsy in breast cancer patients Shishuai Wen1,2,3†, Yiran Liang1†, Xiaoli Kong1, Baofeng Liu4, Tingting Ma1, Yeqing Zhou1, Liyu Jiang1, Xiaoyan Li1 and Qifeng Yang1,5,6* Abstract Background: In light of the extensive application of sentinel lymph node biopsy (SLNB) in clinically node-negative breast cancer patients and the recently investigated failure of SLNB after lumpectomy, it has become important to explore methods for preoperative mapping of sentinel lymph nodes (SLNs) and their lymphatics to direct precise SLNB and improve the identifcation rate of SLNs. Methods: Twenty-seven patients with suspected breast cancer based on the results of the clinical examination and imaging were enrolled in the study. Computed tomographic lymphography (CTLG) followed by CT three-dimensional reconstruction was performed to determine the localization of SLNs and lymphatics on the body surface preopera- tively. Intraoperatively combined staining with methylene blue and indocyanine green was used to evaluate the accuracy and feasibility of CTLG. Results: SLNs and lymphatics from the breast were identifed using CTLG in all patients, and preoperative SLNs and lymphatics localization on the body surface showed a signifcant role in the selection of operative incision and injec- tion points. The accuracy rate of SLN and lymphatic detection by CTLG was 92.6% compared with intraoperatively combined staining. Moreover, preoperative CTLG performed well in SLN number detection, and the accuracy rate was 95.2%. Conclusion: We evaluate the procedure and application of preoperative CTLG in the superfcial localization of SLNs and lymphatics, which may lead to a decreased incidence of cutting of the lymphatics of SLNs and consequently more rapid and accurate SLN detection. -

Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma
Journal of Clinical Medicine Review Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma Ane Gerda Z Eriksson 1,2,* , Ben Davidson 2,3, Pernille Bjerre Trent 1,2, Brynhildur Eyjólfsdóttir 1, Gunn Fallås Dahl 1, Yun Wang 1 and Anne Cathrine Staff 2,4 1 Department of Gynecologic Oncology, Oslo University Hospital, Norwegian Radium Hospital, N-0310 Oslo, Norway; [email protected] (P.B.T.); [email protected] (B.E.); [email protected] (G.F.D.); [email protected] (Y.W.) 2 Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, N-0316 Oslo, Norway; [email protected] (B.D.); [email protected] (A.C.S.) 3 Department of Pathology, Oslo University Hospital, Norwegian Radium Hospital, N-0310 Oslo, Norway 4 Division of Obstetrics and Gynaecology, Oslo University Hospital, Ullevål, N-0424 Oslo, Norway * Correspondence: [email protected] Abstract: Sentinel lymph node (SLN) biopsy has emerged as an alternative staging approach in women with assumed early-stage endometrial carcinoma. Through image-guided surgery and pathologic ultrastaging, the SLN approach is introducing “precision medicine” to the surgical management of gynecologic cancers, providing a comprehensive evaluation of high-yield lymph nodes. This approach improves the surgeons’ ability to detect small-volume metastatic disease while reducing intraoperative and postoperative morbidity associated with lymphadenectomy. Although the majority of clinicians in Europe and the USA have recognized the value of SLN biopsy in endometrial carcinoma and introduced this as part of clinical practice, there is ongoing debate Citation: Eriksson, A.G.Z; Davidson, regarding its role in very low-risk patients as well as in patients at high risk of nodal metastasis. -

Vertebral Fracture and Splenomegaly in a Head
MOLECULAR AND CLINICAL ONCOLOGY 15: 202, 2021 Vertebral fracture and splenomegaly in a head and neck cancer producing granulocyte colony‑stimulating factor: A case report of systemic complications associated with a cytokine‑producing solid tumor NAOYA KITAMURA1, SHINYA SENTO1, ERI SASABE1, KATSUHITO KIYASU2, KOSUKE NAKAJI3, MASANORI DAIBATA4 and TETSUYA YAMAMOTO1 Departments of 1Oral and Maxillofacial Surgery, 2Orthopedic Surgery, 3Radiology, and 4Microbiology and Infection, Kochi Medical School, Kochi University, Kochi 783‑8505, Japan Received March 3, 2021; Accepted June 15, 2021 DOI: 10.3892/mco.2021.2364 Abstract. Granulocyte colony‑stimulating factor (G‑CSF)‑ systemic complications due to the cytokines produced by the producing tumors are rare and are associated with a poor tumor are not well known. The adverse events of recombinant prognosis when they occur in the lungs and the head and neck human G‑CSF (rhG‑CSF) administered to mobilize periph‑ region. Positron emission tomography/computed tomography eral blood stem cells include spinal bone pain and osteoporosis has been reported to show systemic specific accumulation of (with vertebral fractures in severe cases), myocardial infarc‑ fluorodeoxyglucose in these cases, but the systemic compli‑ tion, stroke and splenomegaly (with splenic rupture in severe cations associated with the cytokines produced are not well cases). It has been reported that G‑CSF signaling induces known. We herein present the case of a G‑CSF‑producing osteoporosis by activating osteoclasts through suppression of maxillary sinus squamous cell carcinoma in a 73‑year‑old osteoblast activity (3‑6). Furthermore, the abnormal accumu‑ Japanese woman with a vertebral fracture and splenomegaly. lation of fluorodeoxyglucose (FDG) in the red bone marrow These findings are known severe adverse events of high‑dose on positron emission tomography/computed tomography recombinant human G‑CSF treatment. -
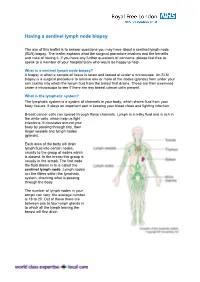
Having a Sentinel Lymph Node Biopsy
Having a sentinel lymph node biopsy The aim of this leaflet is to answer questions you may have about a sentinel lymph node (SLN) biopsy. The leaflet explains what the surgical procedure involves and the benefits and risks of having it. If you have any further questions or concerns, please feel free to speak to a member of your hospital team who would be happy to help. What is a sentinel lymph node biopsy? A biopsy is when a sample of tissue is taken and looked at under a microscope. An SLN biopsy is a surgical procedure to remove one or more of the nodes (glands) from under your arm (axilla) into which the lymph fluid from the breast first drains. These are then examined under a microscope to see if there are any breast cancer cells present. What is the lymphatic system? The lymphatic system is a system of channels in your body, which drains fluid from your body tissues. It plays an important part in keeping your blood clean and fighting infection. Breast cancer cells can spread through these channels. Lymph is a milky fluid and is rich in the white cells, which help us fight infections. It circulates around your body by passing through tiny, then larger vessels and lymph nodes (glands). Each area of the body will drain lymph fluid into certain nodes, usually to the group of nodes which is closest. In the breast this group is usually in the armpit. The first node the fluid drains in to is called the sentinel lymph node. -

Standards for Oncology Registry Entry STORE 2018
STandards for Oncology Registry Entry STORE 2018 Effective for Cases Diagnosed January 1, 2018 STORE STandards for Oncology Registry Entry Released 2018 (Incorporates all updates to Commission on Cancer, National Cancer Database Data standards since FORDS was revised in 2016) Effective for cases diagnosed January 1, 2018 See Appendix A for Updates since FORDS: Revised for 2016. Version 1.0 © 2018 AMERICAN COLLEGE OF SURGEONS All Rights Reserved STORE 2018 Table of Contents Table of Contents Table of Contents ......................................................................................................................... ii Foreword ..................................................................................................................................... 1 FROM “FORDS” TO “STORE” ..................................................................................................................... 1 Preface 2018 ................................................................................................................................ 2 Comorbidities and Complications ............................................................................................................. 2 Revisions to Staging Requirements ........................................................................................................... 2 Staging Data Items No Longer Required for Cases Diagnosed in 2018 and Later (Required for Cases Diagnosed 2017 and Earlier) ................................................................................................................