Additional Chromosome Numbers of American Acanthaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sinopsis De La Familia Acanthaceae En El Perú
Revista Forestal del Perú, 34 (1): 21 - 40, (2019) ISSN 0556-6592 (Versión impresa) / ISSN 2523-1855 (Versión electrónica) © Facultad de Ciencias Forestales, Universidad Nacional Agraria La Molina, Lima-Perú DOI: http://dx.doi.org/10.21704/rfp.v34i1.1282 Sinopsis de la familia Acanthaceae en el Perú A synopsis of the family Acanthaceae in Peru Rosa M. Villanueva-Espinoza1, * y Florangel M. Condo1 Recibido: 03 marzo 2019 | Aceptado: 28 abril 2019 | Publicado en línea: 30 junio 2019 Citación: Villanueva-Espinoza, RM; Condo, FM. 2019. Sinopsis de la familia Acanthaceae en el Perú. Revista Forestal del Perú 34(1): 21-40. DOI: http://dx.doi.org/10.21704/rfp.v34i1.1282 Resumen La familia Acanthaceae en el Perú solo ha sido revisada por Brako y Zarucchi en 1993, desde en- tonces, se ha generado nueva información sobre esta familia. El presente trabajo es una sinopsis de la familia Acanthaceae donde cuatro subfamilias (incluyendo Avicennioideae) y 38 géneros son reconocidos. El tratamiento de cada género incluye su distribución geográfica, número de especies, endemismo y carácteres diagnósticos. Un total de ocho nombres (Juruasia Lindau, Lo phostachys Pohl, Teliostachya Nees, Streblacanthus Kuntze, Blechum P. Browne, Habracanthus Nees, Cylindrosolenium Lindau, Hansteinia Oerst.) son subordinados como sinónimos y, tres especies endémicas son adicionadas para el país. Palabras clave: Acanthaceae, actualización, morfología, Perú, taxonomía Abstract The family Acanthaceae in Peru has just been reviewed by Brako and Zarruchi in 1993, since then, new information about this family has been generated. The present work is a synopsis of family Acanthaceae where four subfamilies (includying Avicennioideae) and 38 genera are recognized. -

Botanischer Garten Der Universität Tübingen
Botanischer Garten der Universität Tübingen 1974 – 2008 2 System FRANZ OBERWINKLER Emeritus für Spezielle Botanik und Mykologie Ehemaliger Direktor des Botanischen Gartens 2016 2016 zur Erinnerung an LEONHART FUCHS (1501-1566), 450. Todesjahr 40 Jahre Alpenpflanzen-Lehrpfad am Iseler, Oberjoch, ab 1976 20 Jahre Förderkreis Botanischer Garten der Universität Tübingen, ab 1996 für alle, die im Garten gearbeitet und nachgedacht haben 2 Inhalt Vorwort ...................................................................................................................................... 8 Baupläne und Funktionen der Blüten ......................................................................................... 9 Hierarchie der Taxa .................................................................................................................. 13 Systeme der Bedecktsamer, Magnoliophytina ......................................................................... 15 Das System von ANTOINE-LAURENT DE JUSSIEU ................................................................. 16 Das System von AUGUST EICHLER ....................................................................................... 17 Das System von ADOLF ENGLER .......................................................................................... 19 Das System von ARMEN TAKHTAJAN ................................................................................... 21 Das System nach molekularen Phylogenien ........................................................................ 22 -

Acanthaceae), a New Chinese Endemic Genus Segregated from Justicia (Acanthaceae)
Plant Diversity xxx (2016) 1e10 Contents lists available at ScienceDirect Plant Diversity journal homepage: http://www.keaipublishing.com/en/journals/plant-diversity/ http://journal.kib.ac.cn Wuacanthus (Acanthaceae), a new Chinese endemic genus segregated from Justicia (Acanthaceae) * Yunfei Deng a, , Chunming Gao b, Nianhe Xia a, Hua Peng c a Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, People's Republic of China b Shandong Provincial Engineering and Technology Research Center for Wild Plant Resources Development and Application of Yellow River Delta, Facultyof Life Science, Binzhou University, Binzhou, 256603, Shandong, People's Republic of China c Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, People's Republic of China article info abstract Article history: A new genus, Wuacanthus Y.F. Deng, N.H. Xia & H. Peng (Acanthaceae), is described from the Hengduan Received 30 September 2016 Mountains, China. Wuacanthus is based on Wuacanthus microdontus (W.W.Sm.) Y.F. Deng, N.H. Xia & H. Received in revised form Peng, originally published in Justicia and then moved to Mananthes. The new genus is characterized by its 25 November 2016 shrub habit, strongly 2-lipped corolla, the 2-lobed upper lip, 3-lobed lower lip, 2 stamens, bithecous Accepted 25 November 2016 anthers, parallel thecae with two spurs at the base, 2 ovules in each locule, and the 4-seeded capsule. Available online xxx Phylogenetic analyses show that the new genus belongs to the Pseuderanthemum lineage in tribe Justi- cieae. -

Hypoestes Aristata (Vahl) Sol
Biol Res 43: 403-409, 2010 BHATT ET AL. Biol Res 43, 2010, 403-409 B403R The foliar trichomes of Hypoestes aristata (Vahl) Sol. ex Roem. & Schult var aristata (Acanthaceae) a widespread medicinal plant species in tropical sub-Saharan Africa: with comments on its possible phylogenetic significance A. Bhatt*, Y. Naidoo and A. Nicholas School of Biological and Conservation Sciences, University of KwaZulu-Natal, Westville Campus, Private Bag X54001, Durban, KZN, 4000, South Africa ABSTRACT The micromorphology of foliar trichomes of Hypoestes aristata var. aristata was studied using stereo, light and scanning microscopy (SEM). This genus belongs to the advanced angiosperm family Acanthaceae, for which few micromorphological leaf studies exist. Results revealed both glandular and non-glandular trichomes, the latter being more abundant on leaf veins, particularly on the abaxial surface of very young leaves. With leaf maturity, the density of non-glandular trichomes decreased. Glandular trichomes were rare and of two types: long-stalked capitate and globose-like peltate trichomes. Capitate trichomes were observed only on the abaxial leaf surface, while peltate trichomes were distributed on both adaxial and abaxial leaf surfaces. Key terms: Acanthaceae, Glandular trichomes, Hypoestes aristata var. aristata, medicinal plant, Scanning electron microscope. INTRODUCTION zygomorphic flowers supported by prominent bracts and producing explosive capsular fruits. Many studies have The Family Acanthaceae is a large and diverse family of further supported the placement of Hypoestes in a smaller dicotyledonous plants comprising about 202 genera and 3520 clade that includes the prominent genus Justicia (McDade species (Judd et al., 2008); although estimates vary from 2600 and Moody 1999). -
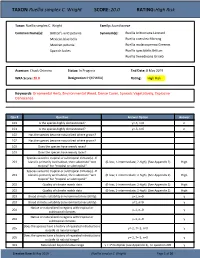
TAXON:Ruellia Simplex C. Wright SCORE:20.0 RATING:High Risk
TAXON: Ruellia simplex C. Wright SCORE: 20.0 RATING: High Risk Taxon: Ruellia simplex C. Wright Family: Acanthaceae Common Name(s): Britton's wild petunia Synonym(s): Ruellia brittoniana Leonard Mexican blue bells Ruellia coerulea Morong Mexican petunia Ruellia malacosperma Greenm. Spanish ladies Ruellia spectabilis Britton Ruellia tweedieana Griseb. Assessor: Chuck Chimera Status: In Progress End Date: 8 May 2019 WRA Score: 20.0 Designation: H(HPWRA) Rating: High Risk Keywords: Ornamental Herb, Environmental Weed, Dense Cover, Spreads Vegetatively, Explosive Dehiscence Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 102 Has the species become naturalized where grown? 103 Does the species have weedy races? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 -

New Species and Transfers Into Justicia (Acanthaceae) James Henrickson California State University, Los Angeles
Aliso: A Journal of Systematic and Evolutionary Botany Volume 12 | Issue 1 Article 6 1988 New Species and Transfers into Justicia (Acanthaceae) James Henrickson California State University, Los Angeles Patricia Hiriart Universidad Nacional Autónoma de México Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Henrickson, James and Hiriart, Patricia (1988) "New Species and Transfers into Justicia (Acanthaceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 12: Iss. 1, Article 6. Available at: http://scholarship.claremont.edu/aliso/vol12/iss1/6 ALISO 12(1), 1988, pp. 45-58 NEW SPECIES AND TRANSFERS INTO JUST/CIA (ACANTHACEAE) JAMES HENRICKSON Department ojBiology California State University Los Angeles, California 90032 AND PATRICIA HIRIART Herbario Nacional, Instituto de Biologia, Universidad Nacional Autonoma de Mexico Apartado Postal 70-367, Delegacion Coyoacan, Mexico, D.F., Mex ico ABSTRACT Justicia medrani and J. zopilot ensis are described as new species while Anisacanthus gonzalezii is transferred into Justicia. The triad all have floral venation similar to red, tubular-flowered species of Just icia, though they differ from most Justicia in their tricolporate pollen with distinct pseudocolpi. In pollen and anther characters they are similar to Anisacanthus and Carlowrightia, but they differ from these in corolla vascularization and anther presentation and from Carlowrightia in corolla size. As the three taxa do not appear to represent a monophyletic group, and as Stearn has placed taxa with similar pollen into what has become a holding genus, Justicia, we include these in Justicia by default until further studies can decipher relat ionships within the genus. -

Unearthing Belowground Bud Banks in Fire-Prone Ecosystems
Unearthing belowground bud banks in fire-prone ecosystems 1 2 3 Author for correspondence: Juli G. Pausas , Byron B. Lamont , Susana Paula , Beatriz Appezzato-da- Juli G. Pausas 4 5 Glo'ria and Alessandra Fidelis Tel: +34 963 424124 1CIDE-CSIC, C. Naquera Km 4.5, Montcada, Valencia 46113, Spain; 2Department of Environment and Agriculture, Curtin Email [email protected] University, PO Box U1987, Perth, WA 6845, Australia; 3ICAEV, Universidad Austral de Chile, Campus Isla Teja, Casilla 567, Valdivia, Chile; 4Depto Ci^encias Biologicas,' Universidade de Sao Paulo, Av P'adua Dias 11., CEP 13418-900, Piracicaba, SP, Brazil; 5Instituto de Bioci^encias, Vegetation Ecology Lab, Universidade Estadual Paulista (UNESP), Av. 24-A 1515, 13506-900 Rio Claro, Brazil Summary To be published in New Phytologist (2018) Despite long-time awareness of the importance of the location of buds in plant biology, research doi: 10.1111/nph.14982 on belowground bud banks has been scant. Terms such as lignotuber, xylopodium and sobole, all referring to belowground bud-bearing structures, are used inconsistently in the literature. Key words: bud bank, fire-prone ecosystems, Because soil efficiently insulates meristems from the heat of fire, concealing buds below ground lignotuber, resprouting, rhizome, xylopodium. provides fitness benefits in fire-prone ecosystems. Thus, in these ecosystems, there is a remarkable diversity of bud-bearing structures. There are at least six locations where belowground buds are stored: roots, root crown, rhizomes, woody burls, fleshy -

Apresentação Do Powerpoint
Yasmin Vidal Hirao Estudos morfológicos e ontogenéticos com inflorescências e flores de Lepidagathis Willd. (Acanthaceae) Morphological and ontogenetic studies with inflorescences and flowers of Lepidagathis Willd. (Acanthaceae) São Paulo Yasmin Vidal Hirao Estudos morfológicos e ontogenéticos com inflorescências e flores de Lepidagathis Willd. (Acanthaceae) Morphological and ontogenetic studies with inflorescences and flowers of Lepidagathis Willd. (Acanthaceae) Dissertação apresentada ao Instituto de Biociências da Universidade de São Paulo, para a obtenção de Título de Mestre em Ciências na Área de Botânica. Orientador: Prof. Dr. Diego Demarco. São Paulo 2015 Hirao, Yasmin Vidal Morphological and ontogenetic studies with inflorescences and flowers of Lepidagathis Willd. (Acanthaceae) 107 páginas Dissertação (Mestrado) – Instituto de Biociências da Universidade de São Paulo. Departamento de Botânica. 1. Anatomy; 2. Development; 3. Evolution; 4. Vascularization; 5. Lamiales; 6. Barlerieae. I. Universidade de São Paulo. Instituto de Biociências. Departamento de Botânica. COMISSÃO JULGADORA _________________________________ _________________________________ Prof(a). Dr(a). Prof(a). Dr(a). _________________________________ Prof. Dr. Diego Demarco (orientador) Dedico este trabalho ao meu vizinho, Totoro, que, em uma das minhas primeiras lembranças botânicas, me ensinou a amar a Natureza. Quando eu flor Quando tu flores E ela flor Nós flores seremos E o mundo florescerá Sandra Braconnot Agradecimentos Primeiramente, gostaria de agradecer -

Baseline Biodiversity Report
FINAL Baseline Biodiversity Survey for Potrero Mason Property Prepared for: County of San Diego Department of Parks and Recreation 5500 Overland Avenue Drive, Suite 410 San Diego, California 92123 Contact: Jennifer Price Prepared by: 605 Third Street Encinitas, California 92024 Contact: Brock Ortega DECEMBER 2012 Printed on 30% post-consumer recycled material. Final Baseline Biodiversity Survey Potrero Mason Property TABLE OF CONTENTS Section Page No. LIST OF ACRONYMS ................................................................................................................ V EXECUTIVE SUMMARY .......................................................................................................VII 1.0 INTRODUCTION..............................................................................................................1 1.1 Purpose of the Report.............................................................................................. 1 1.2 MSCP Context ........................................................................................................ 1 2.0 PROPERTY DESCRIPTION ...........................................................................................9 2.1 Project Location ...................................................................................................... 9 2.2 Geographical Setting ............................................................................................... 9 2.3 Geology and Soils .................................................................................................. -

Horticulture 2016 Newsletter No
Horticulture 2016 Newsletter No. 28 July 12, 2016 2021 Throckmorton Plant Science Cntr. Manhattan, KS 66506 (785) 532-6173 Video of the Week: Dividing Iris UPCOMING EVENTS July 26 K-State Flower Day, Olathe https://www.eventbrite.com/e/k-state-flower-field-day-tickets-26105824223 July 30 K-State Research & Extension Center Horticulture Field Day, Olathe http://www.johnson.k-state.edu/lawn-garden/horticulture-field-day.html August 4 Kansas Turfgrass Field Day, Manhattan https://turffieldday.eventbrite.com Heat Stops Tomatoes from Setting Fruit Temperatures that remain above 75 degrees F at night and day temperatures above 95 degrees F with dry, hot winds will cause poor fruit set on tomatoes. High temperatures interfere with pollen viability and/or cause excessive style growth leading to a lack of pollination. It usually takes about 3 weeks for tomato flowers to develop into fruit large enough to notice that something is wrong and an additional week before tomatoes are full size and ready to start ripening. Though there are "heat-set" tomatoes such as Florida 91, Sun Leaper and Sun Master that will set fruit at higher temperatures, that difference is normally only 2 to 3 degrees. Cooler temperatures will allow flowers to resume fruit set. (Ward Upham) Spider Mites on Tomatoes We have seen some impressive spider mite damage on tomatoes. This is a little surprising considering how little hot and dry weather we have had this summer. Look for stippling on the upper surface of the leaves as well as some fine webbing on the underside of the leaves. -

Machine Learning and Pollination Networks: the Right Flower for Every Bee
MACHINE LEARNING AND POLLINATION NETWORKS: THE RIGHT FLOWER FOR EVERY BEE Aantal woorden: 31 707 Sarah Vanbesien Studentennummer: 01300049 Promotor: prof. dr. Bernard De Baets Copromotor: prof. dr. ir. Guy Smagghe Tutor(s): dr. ir. Michiel Stock, ir. Niels Piot Masterproef voorgelegd voor het behalen van de graad: master in de richting Bio-ingenieurswetenschappen: Milieutechnologie. Academiejaar: 2017 - 2018 De auteur en promotor geven de toelating deze scriptie voor consultatie beschikbaar te stellen en delen ervan te kopiëren voor persoonlijk gebruik. Elk ander gebruik valt onder de beperkingen van het auteursrecht, in het bijzonder met betrekking tot de verplichting uitdrukkelijk de bron te vermelden bij het aanhalen van resultaten uit deze scriptie. The author and promoter give the permission to use this thesis for consultation and to copy parts of it for personal use. Every other use is subject to the copyright laws, more specifically the source must be extensively specified when using results from this thesis. Ghent, June 8, 2018 The promoter, The copromoter, prof. dr. Bernard De Baets prof. dr. ir. Guy Smagghe The tutor, The tutor, The author, dr. ir. Michiel Stock ir. Niels Piot Sarah Vanbesien 4 DANKWOORD ’Oh, een thesis over bloemetjes en bijtjes?’ Het wekt initieël toch net iets senuelere gedachten op dan nodig. Je zou denken dat er wanneer je dan de minder sexy kant van je onderzoek begint uit te leggen veel mensen afhaken, en wel, dat klopt. Gelukkig zit ik in een vakgroep vol mensen die formules en modelleren even interes- sant vinden als ik. Allereerst mijn tutor Michiel, naar jou gaat zonder twijfel mijn grootste dank uit! Iedere dag stond je klaar om mijn ontelbare vragen te beantwoorden. -

Why Continue with Floristic Checklists in Mexico
Botanical Sciences 97 (4): 741-753. 2019 Received: February 15, 2019, accepted: July 15, 2019 DOI: 10.17129/botsci.2174 On line first: December 17, 2019 Taxonomy and Floristics/Taxonomía y Florística Why continue with floristic checklists in Mexico? The case of the Tacaná- Boquerón Priority Terrestrial Region, in the Mexican State of Chiapas ¿Por qué continuar realizando listados florísticos en México? El caso de la Región Terrestre Prioritaria Tacaná-Boquerón, Chiapas Rubén Martínez-Camilo1*, Nayely Martínez-Meléndez2, Manuel Martínez-Meléndez3,4, Miguel Ángel Pérez- Farrera3, and Derio Antonio Jiménez-López1 1 Centro del Cambio Global y La Sustentabilidad A.C., Villahermosa, Tabasco, México. 2 El Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas, México. 3 Herbario Eizi Matuda; Laboratorio de Ecología Evolutiva, Instituto de Ciencias Biológicas, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez, Chiapas, México. 4 Eizia A.C., Tuxtla Gutiérrez, Chiapas, México. * Corresponding author: [email protected] Abstract Background: Some regions of Mexico have been relatively well explored floristically and estimates of the vascular plant richness they contain have been obtained. However, there are still regions that require effort to obtain the most appropriate lists of flora possible that consider both systemization of the information and that benefit from recent botanical explorations. Questions: What is the species richness of vascular plants in the Tacaná-Boquerón Priority Terrestrial Region? What proportion of the species are endemic or included in risk categories? Study sites and dates: Tacaná-Boquerón Priority Terrestrial Region, Chiapas State, Mexico. This region is on the Guatemala border and covers an area of 57,400 ha.