Hypoestes Aristata (Vahl) Sol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

Les Cypéracées Forestières De Côte D'ivoire
G. LOROUGNON LES CYPÉRACEES FORESTI~WES DE C6TE i3’llVOIIRE ÉDITIONS DE L’OFFICE DE LA RECHERCHESCIENTIFKLUE ET TECHNIQUEOUTRE-MER RENSEIGNEMENTS, CONDITIONS DE VENTE Pour tout renseignement, abonnement aux revues pkriodiques, achat d’ouvrages et de cartes, ou demande de catalogue, s’adresser à : I SERVICE CENTRAL DE DOCUMENTATION DE L’ORSTOM 70-74, route d’Aulnay, 93-BONDY (France) - Tout paiement sera effectué par virement postal OU chéque bancaire barré, au nom du Régisseur des Recettes et Dépenses des SSC de I’ORSTOM, 70-74, route d’Aulnay, 93-BONDY; compte courant postal no 9.152-54 PARIS. - Achat au comptant possible à la bibliothèque de I’ORSTOM, 24, rue Bayard, PARIS (83. BEVUES ET BULLETIN DE L’ORSTOM 1. CAHIERS ORSTOM cJ Séries non encore périodiques : - Biologie (3 ou 4 numéros par an) a) Skies trimestrielles : - Géophysique - Entomologie médicale - Océanographie et para.sltologie Prix selon les numéros - Hydrobiologie - Pédolcgie (1 J - Hydrologie - Sciences humaines II. BULLETIN ANALYTIQUE D’ENTOMOLOGIE MEDICALE ET Abonnement : France 95 F; Etranger 115F; le num&o 25 F VETERINAIRE b) Série semestrielle : (Mensuel] - Géologie Abonnement : France 75 F : Etranger 80 F ; le num&ro 40 F Abonnement : France 75 F : Etranger 85 F: le num&ro 8 F (1) Masson et Cie, 120, bd Saint-Germain, Paris-W, kpositalres de cette série à compter du vol. VIII, 1970. Abonnement France : 98F; Etranger : 134 F. Parmi nos publications, nous rappelons : MÉMOIRES : no 7 - ADJANBHOUN (E*) - 1964 - Végétations des sabanes et des roch?rs découverts en Côte d’ivoire Centrale. 250 p. 105 F no20 - CXJlLLAUMET (J.-L.) - 1967 - Recherches sur la végétation et la flore de la région du Bas-Cavally, Côte d’ivoire. -

Sinopsis De La Familia Acanthaceae En El Perú
Revista Forestal del Perú, 34 (1): 21 - 40, (2019) ISSN 0556-6592 (Versión impresa) / ISSN 2523-1855 (Versión electrónica) © Facultad de Ciencias Forestales, Universidad Nacional Agraria La Molina, Lima-Perú DOI: http://dx.doi.org/10.21704/rfp.v34i1.1282 Sinopsis de la familia Acanthaceae en el Perú A synopsis of the family Acanthaceae in Peru Rosa M. Villanueva-Espinoza1, * y Florangel M. Condo1 Recibido: 03 marzo 2019 | Aceptado: 28 abril 2019 | Publicado en línea: 30 junio 2019 Citación: Villanueva-Espinoza, RM; Condo, FM. 2019. Sinopsis de la familia Acanthaceae en el Perú. Revista Forestal del Perú 34(1): 21-40. DOI: http://dx.doi.org/10.21704/rfp.v34i1.1282 Resumen La familia Acanthaceae en el Perú solo ha sido revisada por Brako y Zarucchi en 1993, desde en- tonces, se ha generado nueva información sobre esta familia. El presente trabajo es una sinopsis de la familia Acanthaceae donde cuatro subfamilias (incluyendo Avicennioideae) y 38 géneros son reconocidos. El tratamiento de cada género incluye su distribución geográfica, número de especies, endemismo y carácteres diagnósticos. Un total de ocho nombres (Juruasia Lindau, Lo phostachys Pohl, Teliostachya Nees, Streblacanthus Kuntze, Blechum P. Browne, Habracanthus Nees, Cylindrosolenium Lindau, Hansteinia Oerst.) son subordinados como sinónimos y, tres especies endémicas son adicionadas para el país. Palabras clave: Acanthaceae, actualización, morfología, Perú, taxonomía Abstract The family Acanthaceae in Peru has just been reviewed by Brako and Zarruchi in 1993, since then, new information about this family has been generated. The present work is a synopsis of family Acanthaceae where four subfamilies (includying Avicennioideae) and 38 genera are recognized. -

Index Seminum 2007
Conservatoire et Jardin botaniques Ville de Genève Département municipal des affaires culturelles Delectus Seminum Horti Genevensis 2007-2008 Knautia arvensis (L.) Coulter Delectus seminum quae Hortus genevensis pro mutua commutatione offert anno 2007-2008 Editions des Conservatoire et Jardin botaniques Directeu r: Dr Pierre-André Loizeau Jardinier-che f: Alexandre Breda La récolte des graines s’est effectuée sous la responsabilité de MM. Semina Robert Braito, chef de culture Fréderic Bieri, sous-chef de culture in horto lecta . 6 e plantis spontaneis allata . 15 Traitement informatique des données: Raoul Palese Semina e plantis spontaneis allata 2006 - 2007 - 2008 Couverture: Matthieu Berthod Réalisation technique: Gérard Schilling Caroline Fischer Index Seminum Case postale 60 CH – 1292 Chambésy/Genève Suisse [email protected] Genève, décembre 2007 INFORMATIONS GÉNÉRALES La récolte des graines proposées dans notre Index Seminum se fait dans le respect des lois cantonales et fédérales de protection de la flore, et de la Liste Rouge des plantes menacées de Suisse. La Liste Rouge 2002 des fougères et plantes à fleurs menacées de Suisse comprend la liste de toutes les espèces indigènes et néophytes, accompagnées de la catégorie de menace qui leur a été attribuée sur la base des critères de l’Union Internationale Conservation de la Nature (UICN). C’est pour cette raison que nous nous appliquons à ne pas récolter les plantes qui ont les statuts de protection CR (au bord de l’extinction), EN ( en danger), VU (vulnérable). De plus les plantes sont récoltées en dehors des zones protégées, réserves naturelles et parc national. Coordonnées géographiques des CJB – Latitude: 46°13’N, longitude: 6°8’E Altitude: 382 m Données climatiques (Genève-Cointrin, altitude 420 m) Période 1962 – 1991: GENERAL INFORMATION Température minimum absolue ................................................................................................ -

Towards Resolving Lamiales Relationships
Schäferhoff et al. BMC Evolutionary Biology 2010, 10:352 http://www.biomedcentral.com/1471-2148/10/352 RESEARCH ARTICLE Open Access Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences Bastian Schäferhoff1*, Andreas Fleischmann2, Eberhard Fischer3, Dirk C Albach4, Thomas Borsch5, Günther Heubl2, Kai F Müller1 Abstract Background: In the large angiosperm order Lamiales, a diverse array of highly specialized life strategies such as carnivory, parasitism, epiphytism, and desiccation tolerance occur, and some lineages possess drastically accelerated DNA substitutional rates or miniaturized genomes. However, understanding the evolution of these phenomena in the order, and clarifying borders of and relationships among lamialean families, has been hindered by largely unresolved trees in the past. Results: Our analysis of the rapidly evolving trnK/matK, trnL-F and rps16 chloroplast regions enabled us to infer more precise phylogenetic hypotheses for the Lamiales. Relationships among the nine first-branching families in the Lamiales tree are now resolved with very strong support. Subsequent to Plocospermataceae, a clade consisting of Carlemanniaceae plus Oleaceae branches, followed by Tetrachondraceae and a newly inferred clade composed of Gesneriaceae plus Calceolariaceae, which is also supported by morphological characters. Plantaginaceae (incl. Gratioleae) and Scrophulariaceae are well separated in the backbone grade; Lamiaceae and Verbenaceae appear in distant clades, while the recently described Linderniaceae are confirmed to be monophyletic and in an isolated position. Conclusions: Confidence about deep nodes of the Lamiales tree is an important step towards understanding the evolutionary diversification of a major clade of flowering plants. The degree of resolution obtained here now provides a first opportunity to discuss the evolution of morphological and biochemical traits in Lamiales. -

Central African Biomes and Forest Succession Stages Derived from Modern Pollen Data and Plant Functional Types J
Central African biomes and forest succession stages derived from modern pollen data and plant functional types J. Lebamba, A. Ngomanda, A. Vincens, D. Jolly, C. Favier, H. Elenga, I. Bentaleb To cite this version: J. Lebamba, A. Ngomanda, A. Vincens, D. Jolly, C. Favier, et al.. Central African biomes and forest succession stages derived from modern pollen data and plant functional types. Climate of the Past, European Geosciences Union (EGU), 2009, 5 (3), pp.403-429. 10.5194/cp-5-403-2009. hal-03197644 HAL Id: hal-03197644 https://hal.archives-ouvertes.fr/hal-03197644 Submitted on 14 Apr 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Clim. Past, 5, 403–429, 2009 www.clim-past.net/5/403/2009/ Climate © Author(s) 2009. This work is distributed under of the Past the Creative Commons Attribution 3.0 License. Central African biomes and forest succession stages derived from modern pollen data and plant functional types J. Lebamba1, A. Ngomanda2, A. Vincens3, D. Jolly1,†, -

Acanthaceae), a New Chinese Endemic Genus Segregated from Justicia (Acanthaceae)
Plant Diversity xxx (2016) 1e10 Contents lists available at ScienceDirect Plant Diversity journal homepage: http://www.keaipublishing.com/en/journals/plant-diversity/ http://journal.kib.ac.cn Wuacanthus (Acanthaceae), a new Chinese endemic genus segregated from Justicia (Acanthaceae) * Yunfei Deng a, , Chunming Gao b, Nianhe Xia a, Hua Peng c a Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, People's Republic of China b Shandong Provincial Engineering and Technology Research Center for Wild Plant Resources Development and Application of Yellow River Delta, Facultyof Life Science, Binzhou University, Binzhou, 256603, Shandong, People's Republic of China c Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, People's Republic of China article info abstract Article history: A new genus, Wuacanthus Y.F. Deng, N.H. Xia & H. Peng (Acanthaceae), is described from the Hengduan Received 30 September 2016 Mountains, China. Wuacanthus is based on Wuacanthus microdontus (W.W.Sm.) Y.F. Deng, N.H. Xia & H. Received in revised form Peng, originally published in Justicia and then moved to Mananthes. The new genus is characterized by its 25 November 2016 shrub habit, strongly 2-lipped corolla, the 2-lobed upper lip, 3-lobed lower lip, 2 stamens, bithecous Accepted 25 November 2016 anthers, parallel thecae with two spurs at the base, 2 ovules in each locule, and the 4-seeded capsule. Available online xxx Phylogenetic analyses show that the new genus belongs to the Pseuderanthemum lineage in tribe Justi- cieae. -

Report of Two Spontaneous, Rare Phenotypic Traits in the Genus Phillyrea L
Flora Montiberica 70: 80-86 (III-2018). ISSN: 1138-5952, edic. digital: 1988-799X REPORT OF TWO SPONTANEOUS, RARE PHENOTYPIC TRAITS IN THE GENUS PHILLYREA L. José Luis MEDINA-GAVILÁN SOCEAMB, Sociedad de Estudios Ambientales. C/Perú, 4, 1ª planta. 41100-Coria del Río (Sevilla). [email protected] ABSTRACT: In this note, I document two previously unreported, spontane- ous and exceptionally rare phenotypic expressions affecting reproductive traits in adult plants of the Mediterranean genus Phillyrea (Oleaceae): (i) a morph with an abnormally elongated stigma and lobes transformed in two long branches (i.e. deeper-stigma phenotype), detected in a population of Phillyrea latifolia L. from NE Spain, and (ii) a morph with fruits lacking anthocyanins (i.e. colourless-fruit phenotype), in a population of Phillyrea angustifolia L. from SW Spain. Both phenotypes occurred at a very low frequency within their respective populations. Despite this, the novel traits acquired are discussed in an eco-evolutionary con- text, revealing their potential use as a study model, limited but suggestive, to test adaptive hypothesis in natural conditions. Keywords: phenotypic expression; adaptive value; anemophilous syndrome; selection mediated by frugivorous birds. RESUMEN: Descripción de dos caracteres fenotípicos, raros y espontáneos en el género mediterráneo Phillyrea L. En esta nota documento la presencia de dos expresiones fenotípicas desconocidas, espontáneas y excepcionalmente raras, que afectan a caracteres reproductivos en plantas adultas del género mediterráneo Phillyrea (Oleaceae): (i) un morfo con un estigma anormalmente largo y lóbulos transformados en dos ramas (i.e. fenotipo de estigma profundo), detectado en una población de Phillyrea latifolia L. en el noreste de España, y (ii) un morfo con frutos carentes de antocianinas (i.e. -

March 20, 2013
Plant Availability List – March 20, 2013 Note: A price change has been in effect with the launch of the new web site on March 15, 2011. I will be reworking the web site during the winter of 2012 - 2013 Propagation from cuttings usually takes 2 weeks to root. Colored dots indicate forecasted availability. Newly struck cuttings are marked by M. Newly potted cuttings are marked with a blue dot M and will be ready in 10 - 21 days, depending on variety and temperatures. Recently re-acquired and new stock plants are marked by a green dot M , and some production will be ongoing through winter, but mainly in spring of 2013. Replacement stock plants I need to find a source of are marked by a magenta dot M Those items without any dot are mostly plants that I have as established stock plants. More replacements will be coming. I am restricting distribution of plants labeled PP and especially PPAF to comply with royalty agreements All plants from 2012 stock are in need of repotting or setting out. I will try to issue new availability lists from autumn through spring at least every 4 to 5 weeks. The rare seed plants will be producing new stock plants with some production in sometime in spring 2013. Salvia adenophora out of stockM Salvia coccinea Black & Rouge out of stock Salvia africana out of stock Salvia coccinea Brenthurst out of stockM Salvia albicaulis out of stockM Salvia coccinea Lavender out of stock Salvia albicaulis x africana out of stockM Salvia coccinea Pink Lavender out of stock Salvia algeriensis out of stock Salvia concolor (S. -
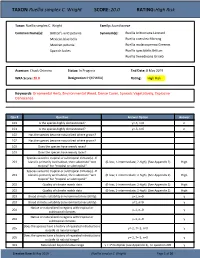
TAXON:Ruellia Simplex C. Wright SCORE:20.0 RATING:High Risk
TAXON: Ruellia simplex C. Wright SCORE: 20.0 RATING: High Risk Taxon: Ruellia simplex C. Wright Family: Acanthaceae Common Name(s): Britton's wild petunia Synonym(s): Ruellia brittoniana Leonard Mexican blue bells Ruellia coerulea Morong Mexican petunia Ruellia malacosperma Greenm. Spanish ladies Ruellia spectabilis Britton Ruellia tweedieana Griseb. Assessor: Chuck Chimera Status: In Progress End Date: 8 May 2019 WRA Score: 20.0 Designation: H(HPWRA) Rating: High Risk Keywords: Ornamental Herb, Environmental Weed, Dense Cover, Spreads Vegetatively, Explosive Dehiscence Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 102 Has the species become naturalized where grown? 103 Does the species have weedy races? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 -

New Species and Transfers Into Justicia (Acanthaceae) James Henrickson California State University, Los Angeles
Aliso: A Journal of Systematic and Evolutionary Botany Volume 12 | Issue 1 Article 6 1988 New Species and Transfers into Justicia (Acanthaceae) James Henrickson California State University, Los Angeles Patricia Hiriart Universidad Nacional Autónoma de México Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Henrickson, James and Hiriart, Patricia (1988) "New Species and Transfers into Justicia (Acanthaceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 12: Iss. 1, Article 6. Available at: http://scholarship.claremont.edu/aliso/vol12/iss1/6 ALISO 12(1), 1988, pp. 45-58 NEW SPECIES AND TRANSFERS INTO JUST/CIA (ACANTHACEAE) JAMES HENRICKSON Department ojBiology California State University Los Angeles, California 90032 AND PATRICIA HIRIART Herbario Nacional, Instituto de Biologia, Universidad Nacional Autonoma de Mexico Apartado Postal 70-367, Delegacion Coyoacan, Mexico, D.F., Mex ico ABSTRACT Justicia medrani and J. zopilot ensis are described as new species while Anisacanthus gonzalezii is transferred into Justicia. The triad all have floral venation similar to red, tubular-flowered species of Just icia, though they differ from most Justicia in their tricolporate pollen with distinct pseudocolpi. In pollen and anther characters they are similar to Anisacanthus and Carlowrightia, but they differ from these in corolla vascularization and anther presentation and from Carlowrightia in corolla size. As the three taxa do not appear to represent a monophyletic group, and as Stearn has placed taxa with similar pollen into what has become a holding genus, Justicia, we include these in Justicia by default until further studies can decipher relat ionships within the genus. -

Acanthaceae and Asteraceae Family Plants Used by Folk Medicinal Practitioners for Treatment of Malaria in Chittagong and Sylhet Divisions of Bangladesh
146 American-Eurasian Journal of Sustainable Agriculture, 6(3): 146-152, 2012 ISSN 1995-0748 ORIGINAL ARTICLE Acanthaceae and Asteraceae family plants used by folk medicinal practitioners for treatment of malaria in Chittagong and Sylhet Divisions of Bangladesh Md. Tabibul Islam, Protiva Rani Das, Mohammad Humayun Kabir, Shakila Akter, Zubaida Khatun, Md. Megbahul Haque, Md. Saiful Islam Roney, Rownak Jahan, Mohammed Rahmatullah Faculty of Life Sciences, University of Development Alternative, Dhanmondi, Dhaka-1205, Bangladesh Md. Tabibul Islam, Protiva Rani Das, Mohammad Humayun Kabir, Shakila Akter, Zubaida Khatun, Md. Megbahul Haque, Md. Saiful Islam Roney, Rownak Jahan, Mohammed Rahmatullah: Acanthaceae and Asteraceae family plants used by folk medicinal practitioners for treatment of malaria in Chittagong and Sylhet Divisions of Bangladesh ABSTRACT Malaria is a debilitating disease causing high mortality rates among men and women if not treated properly. The disease is prevalent in many countries of the world with the most prevalence noted among the sub-Saharan countries, where it is in an epidemic form. The disease is classified as hypo-endemic in Bangladesh with the southeast and the northeastern regions of the country having the most malaria-affected people. The rural people suffer most from malaria, and they rely on folk medicinal practitioners for treatment, who administer various plant species for treatment of the disease as well as associated symptoms like pain and fever. Plant species have always formed the richest sources of anti-malarial drugs, the most notable being quinine and artemisinin. However, quinine has developed drug-resistant vectors and artemisinin is considered by some to developing initial resistance, particularly in China, where it has been used for thousands of years to combat malaria.