Compositions and Comparison of the Leaf and Stem Essential Oils from Nigerian Hypoestes Phyllostachya ‘ ’ Rosea P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Relationships Among the Mangrove Species of Acanthaceae Found in Indian Sundarban, As Revealed by RAPD Analysis
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2015, 6(3):179-184 ISSN: 0976-8610 CODEN (USA): AASRFC Phylogenetic relationships among the mangrove species of Acanthaceae found in Indian Sundarban, as revealed by RAPD analysis Surya Shekhar Das 1, Swati Das (Sur) 2 and Parthadeb Ghosh* 1Department of Botany, Bolpur College, Birbhum, West Bengal, India 2Department of Botany, Nabadwip Vidyasagar College, Nadia, West Bengal, India _____________________________________________________________________________________________ ABSTRACT RAPD markers were successfully used to identify and differentiate all the five species of Acanthaceae found in the mangrove forest of Indian Sundarban, to assess the extent of interspecific genetic diversity among them, to reveal their molecular phylogeny and to throw some light on the systematic position of Avicennia. The dendrogram reveals that the five species under study exhibits an overall similarity of 60.7%. Avicennia alba and A. officinalis (cluster C1) have very close relationship between them and share a common node in the dendrogram at a 73.3% level of similarity. Avicennia marina and Acanthus ilicifolius (cluster C2) also have close relationship between them as evident by a common node in the dendrogram at 71.8% level of similarity. Acanthus volubilis showed 68.1% similarity with cluster C1 and 60.7% similarity with cluster C2. Our study also supported the view of placing Avicennia under Acanthaceae. Regarding the relative position of Avicennia within Acanthaceae, it was shown to be very close to Acanthoideae. In comparison to other species, A. marina showed most genetic variability, suggesting utilization of this species over others for breeding programme and as source material in in situ conservation programmes. -

Acanthaceae), a New Chinese Endemic Genus Segregated from Justicia (Acanthaceae)
Plant Diversity xxx (2016) 1e10 Contents lists available at ScienceDirect Plant Diversity journal homepage: http://www.keaipublishing.com/en/journals/plant-diversity/ http://journal.kib.ac.cn Wuacanthus (Acanthaceae), a new Chinese endemic genus segregated from Justicia (Acanthaceae) * Yunfei Deng a, , Chunming Gao b, Nianhe Xia a, Hua Peng c a Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, People's Republic of China b Shandong Provincial Engineering and Technology Research Center for Wild Plant Resources Development and Application of Yellow River Delta, Facultyof Life Science, Binzhou University, Binzhou, 256603, Shandong, People's Republic of China c Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, People's Republic of China article info abstract Article history: A new genus, Wuacanthus Y.F. Deng, N.H. Xia & H. Peng (Acanthaceae), is described from the Hengduan Received 30 September 2016 Mountains, China. Wuacanthus is based on Wuacanthus microdontus (W.W.Sm.) Y.F. Deng, N.H. Xia & H. Received in revised form Peng, originally published in Justicia and then moved to Mananthes. The new genus is characterized by its 25 November 2016 shrub habit, strongly 2-lipped corolla, the 2-lobed upper lip, 3-lobed lower lip, 2 stamens, bithecous Accepted 25 November 2016 anthers, parallel thecae with two spurs at the base, 2 ovules in each locule, and the 4-seeded capsule. Available online xxx Phylogenetic analyses show that the new genus belongs to the Pseuderanthemum lineage in tribe Justi- cieae. -

Hypoestes Aristata (Vahl) Sol
Biol Res 43: 403-409, 2010 BHATT ET AL. Biol Res 43, 2010, 403-409 B403R The foliar trichomes of Hypoestes aristata (Vahl) Sol. ex Roem. & Schult var aristata (Acanthaceae) a widespread medicinal plant species in tropical sub-Saharan Africa: with comments on its possible phylogenetic significance A. Bhatt*, Y. Naidoo and A. Nicholas School of Biological and Conservation Sciences, University of KwaZulu-Natal, Westville Campus, Private Bag X54001, Durban, KZN, 4000, South Africa ABSTRACT The micromorphology of foliar trichomes of Hypoestes aristata var. aristata was studied using stereo, light and scanning microscopy (SEM). This genus belongs to the advanced angiosperm family Acanthaceae, for which few micromorphological leaf studies exist. Results revealed both glandular and non-glandular trichomes, the latter being more abundant on leaf veins, particularly on the abaxial surface of very young leaves. With leaf maturity, the density of non-glandular trichomes decreased. Glandular trichomes were rare and of two types: long-stalked capitate and globose-like peltate trichomes. Capitate trichomes were observed only on the abaxial leaf surface, while peltate trichomes were distributed on both adaxial and abaxial leaf surfaces. Key terms: Acanthaceae, Glandular trichomes, Hypoestes aristata var. aristata, medicinal plant, Scanning electron microscope. INTRODUCTION zygomorphic flowers supported by prominent bracts and producing explosive capsular fruits. Many studies have The Family Acanthaceae is a large and diverse family of further supported the placement of Hypoestes in a smaller dicotyledonous plants comprising about 202 genera and 3520 clade that includes the prominent genus Justicia (McDade species (Judd et al., 2008); although estimates vary from 2600 and Moody 1999). -

SAPIA Newsletters Are Posted at WIP and Can Be Downloaded Free of Charge Emerging Invasive Grasses 3
SAPIA NEWS SOUTHERN AFRICAN PLANT INVADERS ATLAS April 2008 ARC-Plant Protection Research Institute No. 7 SAPIA phase II—reminders SAPIA phase II focuses on emerging invasive species. The public is invited to submit records for the species that have been highlighted in the SAPIA newsletters as well as any other species that they may be aware of. Please only submit records of alien plant species that are growing beyond the confines of cultivation e.g. along roads, rivers, in urban open space, in disturbed or undisturbed Inside this issue: natural areas. If you are uncertain about the identification of a plant then send a dried, pressed specimen preferably with flowers and/or fruits to Lesley Henderson. Good digital photos are also acceptable. SAPIA phase II—reminders 1 SAPIA needs your support! Please submit records to the Weeds and Invasive Plants website Pompom weed— www.agis.agric.za/wip 1 & 2 update Public participation is vital to the SAPIA II project. If you should have any trouble in submitting records at the WIP site then rather e-mail them to Lesley Henderson at Progress with legislation 2 [email protected] All the SAPIA Newsletters are posted at WIP and can be downloaded free of charge Emerging invasive grasses 3 Emerging ornamental weeds: Pompom weed update Lindenleaf sage 4 Creeping knotweed Polka-dot-plant Pompom weed ( Campuloclinium macrocephalum ) continues to expand its range. SAPIA surveys have added many new localities in Mpumalanga (see map overleaf). The National Road Agency in North West detected (and treated) pompom You are invited to participate in the weed along the N14 near Barberspan SAPIA phase II project. -

Acanthaceae and Asteraceae Family Plants Used by Folk Medicinal Practitioners for Treatment of Malaria in Chittagong and Sylhet Divisions of Bangladesh
146 American-Eurasian Journal of Sustainable Agriculture, 6(3): 146-152, 2012 ISSN 1995-0748 ORIGINAL ARTICLE Acanthaceae and Asteraceae family plants used by folk medicinal practitioners for treatment of malaria in Chittagong and Sylhet Divisions of Bangladesh Md. Tabibul Islam, Protiva Rani Das, Mohammad Humayun Kabir, Shakila Akter, Zubaida Khatun, Md. Megbahul Haque, Md. Saiful Islam Roney, Rownak Jahan, Mohammed Rahmatullah Faculty of Life Sciences, University of Development Alternative, Dhanmondi, Dhaka-1205, Bangladesh Md. Tabibul Islam, Protiva Rani Das, Mohammad Humayun Kabir, Shakila Akter, Zubaida Khatun, Md. Megbahul Haque, Md. Saiful Islam Roney, Rownak Jahan, Mohammed Rahmatullah: Acanthaceae and Asteraceae family plants used by folk medicinal practitioners for treatment of malaria in Chittagong and Sylhet Divisions of Bangladesh ABSTRACT Malaria is a debilitating disease causing high mortality rates among men and women if not treated properly. The disease is prevalent in many countries of the world with the most prevalence noted among the sub-Saharan countries, where it is in an epidemic form. The disease is classified as hypo-endemic in Bangladesh with the southeast and the northeastern regions of the country having the most malaria-affected people. The rural people suffer most from malaria, and they rely on folk medicinal practitioners for treatment, who administer various plant species for treatment of the disease as well as associated symptoms like pain and fever. Plant species have always formed the richest sources of anti-malarial drugs, the most notable being quinine and artemisinin. However, quinine has developed drug-resistant vectors and artemisinin is considered by some to developing initial resistance, particularly in China, where it has been used for thousands of years to combat malaria. -

Downloaded and Set As out Groups Genes
Alzahrani et al. BMC Genomics (2020) 21:393 https://doi.org/10.1186/s12864-020-06798-2 RESEARCH ARTICLE Open Access Complete chloroplast genome sequence of Barleria prionitis, comparative chloroplast genomics and phylogenetic relationships among Acanthoideae Dhafer A. Alzahrani1, Samaila S. Yaradua1,2*, Enas J. Albokhari1,3 and Abidina Abba1 Abstract Background: The plastome of medicinal and endangered species in Kingdom of Saudi Arabia, Barleria prionitis was sequenced. The plastome was compared with that of seven Acanthoideae species in order to describe the plastome, spot the microsatellite, assess the dissimilarities within the sampled plastomes and to infer their phylogenetic relationships. Results: The plastome of B. prionitis was 152,217 bp in length with Guanine-Cytosine and Adenine-Thymine content of 38.3 and 61.7% respectively. It is circular and quadripartite in structure and constitute of a large single copy (LSC, 83, 772 bp), small single copy (SSC, 17, 803 bp) and a pair of inverted repeat (IRa and IRb 25, 321 bp each). 131 genes were identified in the plastome out of which 113 are unique and 18 were repeated in IR region. The genome consists of 4 rRNA, 30 tRNA and 80 protein-coding genes. The analysis of long repeat showed all types of repeats were present in the plastome and palindromic has the highest frequency. A total number of 98 SSR were also identified of which mostly were mononucleotide Adenine-Thymine and are located at the non coding regions. Comparative genomic analysis among the plastomes revealed that the pair of the inverted repeat is more conserved than the single copy region. -

ACANTHACEAE 爵床科 Jue Chuang Ke Hu Jiaqi (胡嘉琪 Hu Chia-Chi)1, Deng Yunfei (邓云飞)2; John R
ACANTHACEAE 爵床科 jue chuang ke Hu Jiaqi (胡嘉琪 Hu Chia-chi)1, Deng Yunfei (邓云飞)2; John R. I. Wood3, Thomas F. Daniel4 Prostrate, erect, or rarely climbing herbs (annual or perennial), subshrubs, shrubs, or rarely small trees, usually with cystoliths (except in following Chinese genera: Acanthus, Blepharis, Nelsonia, Ophiorrhiziphyllon, Staurogyne, and Thunbergia), isophyllous (leaf pairs of equal size at each node) or anisophyllous (leaf pairs of unequal size at each node). Branches decussate, terete to angular in cross-section, nodes often swollen, sometimes spinose with spines derived from reduced leaves, bracts, and/or bracteoles. Stipules absent. Leaves opposite [rarely alternate or whorled]; leaf blade margin entire, sinuate, crenate, dentate, or rarely pinnatifid. Inflo- rescences terminal or axillary spikes, racemes, panicles, or dense clusters, rarely of solitary flowers; bracts 1 per flower or dichasial cluster, large and brightly colored or minute and green, sometimes becoming spinose; bracteoles present or rarely absent, usually 2 per flower. Flowers sessile or pedicellate, bisexual, zygomorphic to subactinomorphic. Calyx synsepalous (at least basally), usually 4- or 5-lobed, rarely (Thunbergia) reduced to an entire cupular ring or 10–20-lobed. Corolla sympetalous, sometimes resupinate 180º by twisting of corolla tube; tube cylindric or funnelform; limb subactinomorphic (i.e., subequally 5-lobed) or zygomorphic (either 2- lipped with upper lip subentire to 2-lobed and lower lip 3-lobed, or rarely 1-lipped with 3 lobes); lobes ascending or descending cochlear, quincuncial, contorted, or open in bud. Stamens epipetalous, included in or exserted from corolla tube, 2 or 4 and didyna- mous; filaments distinct, connate in pairs, or monadelphous basally via a sheath (Strobilanthes); anthers with 1 or 2 thecae; thecae parallel to perpendicular, equally inserted to superposed, spherical to linear, base muticous or spurred, usually longitudinally dehis- cent; staminodes 0–3, consisting of minute projections or sterile filaments. -

The Acanthaceae, Derived from Acanthus Are
Vol. 7(36), pp. 2707-2713, 25 September, 2013 DOI: 10.5897/JMPR2013.5194 ISSN 1996-0875 ©2013 Academic Journals Journal of Medicinal Plants Research http://www.academicjournals.org/JMPR Full Length Research Paper Ethnobotany of Acanthaceae in the Mount Cameroon region Fongod A.G.N*, Modjenpa N.B. and Veranso M.C Department of Botany Plant Physiology, University of Buea, P.O Box 63, Buea. Cameroon. Accepted 2 September, 2013 An ethnobotanical survey was carried out in the Mount Cameroon area, southwest region of Cameroon to determine the uses of different species of the Acanthaceae. An inventory of identified Acanthaceaes used by different individuals and traditional medical practitioners (TMPs) was established from information gathered through the show-and-tell/semi-structured method and interviews during field expeditions. Sixteen villages were selected for this research: Munyenge, Mundongo, Ekona, Lelu, Bokoso, Bafia. Bakingili, Ekonjo, Mapanja, Batoke, Wututu, Idenau, Njongi, Likoko, Bokwango and Upper farms. The study yielded 18 plant species used for treating twenty five different diseases and 16 species with ornamental potentials out of the Acanthaceaes identified. Results revealed that 76% of species are used medicinally, while 34% are employed or used for food, rituals, forage and hunting. The leaves of these species are the most commonly used plant parts. The species with the highest frequency of use was Eremomastax speciosa (Hotsch.) with 29 respondents followed by Acanthus montanus (Nes.) T. Anders. The study reveals the medicinal and socio-cultural uses of the Acanthaceaes in the Mount Cameroon Region and a need for proper investigation of the medicinal potentials of these plants. -

Avicennia Germinans (Acanthaceae) Gaining New Ground in Southeast Texas?
Rosen, D.J. and S. Zamirpour. 2014. Avicennia germinans (Acanthaceae) gaining ground in southeast Texas? Phytoneuron 2014-74: 1–4. Published 22 July 2014. ISSN 2153 733X AVICENNIA GERMINANS (ACANTHACEAE) GAINING NEW GROUND IN SOUTHEAST TEXAS? DAVID J. ROSEN and SIAVASH ZAMIRPOUR Lee College Department of Biology P.O. Box 818 Baytown, Texas 77522 [email protected] ABSTRACT Avicennia germinans is documented for the first time from Brazoria County in southeast Texas. It perhaps indicates an expanding range for the species in Texas. Avicennia germinans (L.) L. (black mangrove) is a tropical and subtropical maritime shrub to small tree distributed along tidal shores from Texas to Florida, Bermuda, the Bahama Islands, both coasts of Mexico and Central America, the West Indies, and the coasts of Brazil, Peru, and West Africa (Correll & Johnston 1970; Godfrey & Wooten 1981). The northern distributional limit of Avicennia germinans has been suggested to be determined by the frequency and duration of freezing temperatures (Sherrod & McMillan 1981). In the Gulf of Mexico, A. germinans sees its northernmost limit in Texas. Herbarium records occur from the South Texas coastal counties of Aransas, Calhoun, Cameron, Kleberg, Nueces, and San Patricio (Fig. 1) with well established stands at three sites (Sherrod & McMillan 1981). The species does not occur northward again until Galveston and Jefferson (the northernmost limit) counties in southeast Texas (Fig. 1). Although probably a waif in Jefferson County, as reported by Sherrod and McMillan (1981), Guo et al. (2009), and suggested by few herbarium collections, a well established population occurs in tidal marsh adjacent to "The Lagoon" on northeast Galveston Island (Rosen, personal observation). -
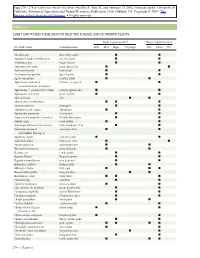
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum -

Regional Floras: Increasing Their Value While Reducing Their Cost
BIO Web of Conferences 24, 00010 (2020) https://doi.org/10.1051/bioconf/20202400010 International Conferences “Plant Diversity: Status, Trends, Conservation Concept” 2020 Regional floras: increasing their value while reducing their cost Mary E. Barkworth1*, Marina V. Olonova2, Polina D. Gudkova3, Zahid Ullah4, and Curtis Dyreson5 1Intermountain Herbarium, Department of Biology, Utah State University, Logan, Utah 84322-5305, USA 2Department of Botany, Altai State University, Russia 3Department of Environmental Management, Biological Institute, Tomsk State University, Russia 4Center for Plant Science and Biodiversity, University of Swat, Khyber Pakhtunkhwa, Pakistan, 5Department of Computer Science, Utah State University, 4205 Old Main Hill, Logan, Utah 84322- 4205, USA Abstract. Regional floras are primary resources for plant identification, an essential step in developing conservation strategies. They also provide students with a scientific window on the plants around them and help them learn botanical terminology, but they are expensive to maintain and publish. We are developing web-accessible updates for different floras, as part of which we are using online resources to help us work more effectively while rapidly providing richer resources. We use KeyBase for sharing dichotomous keys, linking the terminal taxa to subsidiary keys or descriptive taxon pages. Taxon pages are generated in OpenHerbarium which enables integrating specimen and observation data with descriptions, line drawings, and images and displaying maps based on georeferenced specimen data. Its nomenclatural backbone is easily modified to reflect new treatment and can also handle multiple taxonomies. We are examining is the possibility of using a Wikipedia approach to provide a glossary. 1 Introduction Regional floras are incredibly valuable. They enable mapping the distribution of plant diversity, a key step to understanding ecosystems at multiple scales and development of conservation plans. -

Dicliptera Aegyptiaca (Acanthaceae), a New Species from Egypt Supported by Morphological Characters and Rbcl-Based DNA Barcoding Eman M
35 Egypt. J. Bot. Vol. 59, No.2, pp. 475 - 482 (2019) Dicliptera aegyptiaca (Acanthaceae), A New Species from Egypt Supported by Morphological Characters and rbcl-based DNA Barcoding Eman M. Shamso(1), Ahmed S. Fouad(2)# (1)The Herbarium, Botany and Microbiology Department, Faculty of Science, Cairo University, Giza 12613, Egypt; (2)Botany and Microbiology Department, Faculty of Science, Cairo University, Giza 12613, Egypt. ICLIPTERA aegyptiaca, a new species from Red Sea Coast, Egypt, is described and Dillustrated. Diagnostic and morphological characters that distinguish it from its allied species D. paniculata and an identification key for the two species are provided. The new species differs from D. paniculata by having an unbranched stem, a congested inflorescence with dwarf axes 1.5– 5mm long; subsessile cymules with peduncles 0.5– 1mm long. rbcl-DNA barcoding is presented for this new taxon for the first time. Phylogentic tree revealed barcode clusters for the two Dicliptera species and recognized significant interspecific variation between them. D. aegyptiaca clearly formed one clade strongly supported with a bootstrap value of 100%. Based on characters of morphology, pollen and seeds, the new species was recognized as belonging to the genus Dicliptera. On the other hand, DNA barcoding reflected clustering of all Dicliptera spp. in a large clade while D. aegyptiaca formed a non sister clade showing the utility of DNA barcoding for species identification rather than taxonomy. Keywords: Dicliptera aegyptiaca, DNA barcoding, Egypt, Morphology, New species. Introduction Peristrophe are together monophyletic. The genus Dicliptera Juss. comprises about 175 Dicliptera (inclusive of Peristrophe) is species (Daniel, 2009), distributed in tropical characterized by angled stem, opposite leaves, and subtropical countries of Asia, Africa and inflorescence panicle-like cymose, with 2–3 America.