Anhydrite Caso4 C 2001-2005 Mineral Data Publishing, Version 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Geologischen Karte
Kennt ‚H149 fi/M W2 y fl Erläuterungen Geologischen Karte V011 Preußen und benachbarten Bundesstaaten. Herausgegeben von der Königlich Preußischen Geologischen Landesanstalt. Lieferung 187. Blatt Bröckel. Gradabteilung 41, Nr. 30. Geologisch und bodenkundlich bearbeitet durch E. Harhort, H. Monke und l. Stoller. Erläutert durch J. Stoller, der bodenkundliche Teil durch E.’Harhort. Mit einer Übersichtskarte. ~NVV\/\/\I\N\I\N\’W\I\N\M WAI\/W\N\4V\J\ I\/\/' BERLIN. Im Vertrieb bei der Königlichen Geologischen Landesanstalt Berlin N. 4, Invalidenstraße 44. 1916. g v- . fix-‘nv.1 J. m ‚P: h sie. 2’? iii ECA REG I A ALTE; mam. GEURGIAE AUG. Geologische Übersichtskarte der Gegend von Celle. ZF'eu' ee' ' ' 53h: Zeichen—Erklärung Tertiär Diluvium Ei-- JEpfan'ez: fan ‘ In fe-r;who! A”: es cigar 70!? Diluvium ‚i’ffdx‘ä Hocfifl Jung - a"?am: an a“ 212'ms?yawn- gamma: arejnfrei Heim“; Hochfläche gerjqd’äandb edeckung Diluvium TM: and 1'; (Shem qfé Haupfafufe ms?jüngeren [rosz'anssfufen A Huv iu rn Sand u. Sowie} Meere flänsn der gra Een #0:? großem Ffuffäfer Umfang Maßslab 1:200000 ._':;r_„._1.__:=—'x . :__.......—.-.*:.H. Q Fuhflbergf'l‘fir' .-—-——————.- i. Ir I A Lfi- .in:1, 3-5“ . 2lHr ifl' enlw. J.31ullar. Gegend von Celle. Lief. 187 und 191. sua Göttin 207 81 808 3 l Blatt Bröckel. Gradabteilung 41, Nr. 30. Geologisch und bodenkundlich bearbeitet durch E. Harbort, H. Monke und J. Stoller. Erläutert durch l. Stollor, der bodenkundliche Teil durch E. Harbort. ——.—.——— Mit l Übersichtskarte. Bekanntmachung. Jeder Erläuterung liegt eine »Kurze Einführung in das Verständnis der geologisch-agronomischen Karten<<, sowie ein Verzeichnis der bis- herigen Veröffentlichungen der Königlich Preußischen Geologischen Landesanstalt bei. -
Die Gärten Der „Offenen Pforte“ Öffnen Wieder
Die Offene Pforte im Celler Land Gärten & Kultur 2021 www.vhs-celle.de GÄRTEN IN CELLE STADT UND LAND Die Offene Pforte im Celler Land wurde auf Initiative privater Gärtner*innen gegründet und steht heute unter dem Dach der Volks- hochschule (vhs) Celle. Wir heißen schöne Gärten jederzeit herzlich willkommen. Liebe Gartenfreunde, unser Titelbild zeigt es: Wir haben ein verdreh- tes Jahr hinter uns – und wohl auch noch weiter vor uns. Dennoch bleiben wir optimistisch und öffnen für Sie wieder unsere privaten Gärten. Je nach Corona-Lage kann es jedoch kurzfristig zu Aktuelle Ausfall oder Verschiebung der Termine kommen. Infos Aktuelle Infos finden Sie auf der Homepage: www.offene-pforte-celle.de – darunter auch die Hygiene- und Abstandsregeln. Viele unserer Mitglieder konnten ihre Termine im letzten Jahr nicht wahrnehmen, stattdessen haben sie Gästen die Möglichkeit angeboten, Indivi- sich per Telefon anzumelden und nach Abspra- duelle che vorbeizuschauen. Garten- Diese Alternative gilt weiterhin. Achten Sie bitte besuche auf die Hinweise bei den einzelnen Gärten und scheuen Sie sich nicht anzurufen. Wer beim Betrachten unserer Gärten Lust bekommt, seinen eigenen Garten zu öffnen, Machen ist herzlich willlkommen. Rufen Sie an unter Sie mit! (05141) 92 98 20 oder schreiben Sie eine Mail an: [email protected]. Neu dabei sind in diesem Jahr u.a. die Gärten von Sybille Thiele in Oppershausen (21) und Neue Astrid Reschke in Hermannsburg (18), Familie Gärten Adam aus Vorwerk ist mit dabei (2) ebenso wie der Bauerngarten Uetze-Wackerwinkel (15) und der NABU Gemeinschaftgarten (16) in Celle. Schauen Sie auch auf Seite 25: Unsere Mit- glieder Ellen Bielert und Ulrike Tremmel halten Gartenvorträge an der vhs Celle. -

Prolog. Hanna Fueß: Die Chronik Des Landkreises Celle, 15.6. 1946 Bis
Inhalt Einleitung Nachkriegsleben in einem ländlichen Raum. Der Landkreis Celle und die Sammlung Hanna Fueß Auftrag für eine Kriegs- und Nachkriegschronik 9 Zur Biographie von Hanna Fueß 10 Der Landkreis Celle bis zum Ende des Zweiten Weltkrieges ... 13 Die unmittelbare Nachkriegszeit im Landkreis Celle 21 Die Sammlung Hanna Fueß 47 Zur Edition 50 Dokumente Prolog. Hanna Fueß: Die Chronik des Landkreises Celle, 15.6.1946 bis 15.6.1948 53 1. Wolthausen: Wilhelmine Speckhahn, Landwirtin, 16.9.1947 . 58 2. Hassel: Marie Krüger, Altbäuerin, 22.4.1948 65 3. Winsen: Heinrich Bensch, Pastor, 26.6.1947 72 4. Winsen: Walter Redeker, Apotheker, 27.3.1947 74 5. Winsen: Otto Greve, Tiefbauunternehmer, 24.4.1947 79 6. Winsen: Oskar Stillmark, Flüchtlingsobmann, 25.7.1946 .... 82 7. Wietze: Herta Heyer, Gemeindeangestellte, 27.3.1947 85 8. Wietze: Heinrich Höltershinken, Elektromeister, und seine Frau, 28.11.1946 88 9. Ovelgönne: Frieda Glier, Landwirtin, 26.6.1947 90 10. Hambühren: Ferdinand Knoop, Pächter, 27.3.1947 95 11. Hambühren: Irmgard Müller, Flüchtling, 29.1.1948 98 12. Gut Holtau: Hildegard Ziemer, Landwirtin, 27.7.1948 99 13. Boye: Hermann Reinecke, Landwirt, 8.1.1948 102 14. Scheuen: Helmut Rehwinkel und Hermann Garner, Landwirte, 23.7.1946 108 15. Scheuen: Ilse Hemann, Gastwirtin, 15.8.1946 111 16. Groß Hehlen: Heinrich Otte, Lehrer, und seine Frau, 7.8.1946 . 117 17. Garßen: Gustav Sohnemann (sen.), Landwirt, 15.4.1947/16.6.1947 122 6 Inhalt 18. Altenhagen: Albert Knoop, Landwirt, 11.6.1947 130 19. Lachtehausen: Walther Fricke, Müller, 20.5.1947 134 20. Altencelle: Wilhelm Deecke, Landwirt, 1.5.1947 141 21. -

Barite (Barium)
Barite (Barium) Chapter D of Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply Professional Paper 1802–D U.S. Department of the Interior U.S. Geological Survey Periodic Table of Elements 1A 8A 1 2 hydrogen helium 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 lithium beryllium boron carbon nitrogen oxygen fluorine neon 6.94 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 sodium magnesium aluminum silicon phosphorus sulfur chlorine argon 22.99 24.31 3B 4B 5B 6B 7B 8B 11B 12B 26.98 28.09 30.97 32.06 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 potassium calcium scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc gallium germanium arsenic selenium bromine krypton 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.64 74.92 78.96 79.90 83.79 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 rubidium strontium yttrium zirconium niobium molybdenum technetium ruthenium rhodium palladium silver cadmium indium tin antimony tellurium iodine xenon 85.47 87.62 88.91 91.22 92.91 95.96 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 cesium barium hafnium tantalum tungsten rhenium osmium iridium platinum gold mercury thallium lead bismuth polonium astatine radon 132.9 137.3 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.5 204.4 207.2 209.0 (209) (210)(222) 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 -

2012 Bmc Auction Specimens
A SAMPLER OF SELECTED 2017 BMC AUCTION SPECIMENS (2017 Auction Date is Saturday, 21 January) Volume 3 3+ Hematite [Fe 2O3] & Quartz [SiO2] Locality Cleator Moor Iron Mines Cleator Moor West Cumberland Iron Field Cumbria, England, UK Size 13.5 x 9.5 x 7.0 cm 1498 g Donated by Stonetrust Hematite Crystal System: Trigonal Photograph by Mike Haritos Physical Properties Transparency: Subtranslucent to opaque Mohs hardness: 6.5 Density: approx 5.3 gm/cm3 Streak: Red Luster: Metalic Vanadinite [Pb5(VO4)3Cl] var. Endlichite Locality Erupción Mine (Ahumada Mine) Los Lamentos Mountains (Sierra de Los Lamentos) Mun. de Ahumada Chihuahua, Mexico Size 12.0 x 9.5 x 7.0 cm 1134 g Donated by Stonetrust Crystal System: Hexagonal Physical Properties Transparency: Subtranslucent to opaque Mohs hardness: 3.5-4 Photograph by Mike Haritos Density: 6.8 to 7.1 gm/cm3 Streak: Brownish yellow Endlichite, Pb5([V, As]O4)3Cl, is the arsenic rich Luster: Adamantine variety of vanadinite with arsenic atoms (As) substituting for some of the vanadium (V) 2+ Dolomite [CaMg(CO3)2] & Chalcopyrite [CuFe S2] Locality Picher Field Tri-State District Ottawa Co. Oklahoma, USA Size 19.0 x 14.5 x 6.0 cm 1892 g Consigned with Reserve by Stonetrust Dolomite Crystal System: Trigonal Physical Properties Photograph by Mike Haritos Transparency: Transparent, Translucent, Opaque Mohs hardness: 3.5 to 4 Density: 2.8 to 2.9 gm/cm3 Streak: White Luster: Vitreous Calcite [CaCO3] Locality Mexico Size 15.5 x 12.8 x 6.2 cm 1074 g Donated by Stonetrust Calcite Crystal System: Trigonal Physical Properties Transparency: Transparent, Translucent Mohs hardness: 3 Density: 2.71 gm/cm3 Streak: White Luster: Vitreous, Sub-Vitreous, Photograph by Mike Haritos Resinous, Waxy, Pearly Quartz [SiO2], var. -

Aktuelle Mitteilung Aus Dem Rathaus
Energiemuseum Spechtshorn Aktuelle Mitteilung aus dem Rathaus Aufgrund der aktuellen Corona-Lage kann das Rathaus bis auf Weiteres nur nach vorheriger Terminvereinbarung betreten werden. Für eine Terminvereinbarung rufen Sie bitte die zuständige Mitarbeiterin / den zuständigen Mitarbeiter an. Die Telefonnummern finden Sie auf unserer Homepage www.lachendorf.de 2 Ernennung zum Ersten Samtgemeinderat ab dem 1. Januar 2022 An der Spitze der Verwaltung im Rathaus stehen zum Ende des Jahres 2021 wesentliche Änderungen an. Un- ter anderem wird die Erste Samtgemeinderätin Uta Lüßmann am 01.01.2022 in den Ruhestand eintreten. Durch den Rat der Samtgemeinde Lachendorf wurde Herr Eike Christian Hebecker zum Ersten Samtge- meinderat für die Dauer von 8 Jahren und damit zum Nachfolger von Frau Lüßmann gewählt. Herr Hebe- cker ist seit dem 01.03.2010 bei der Samtgemeinde La- chendorf als Leiter des Fachbereiches Finanzen tätig. Er wird das Amt des Ersten Samtgemeinderates zum 01.01.2022 übernehmen. Die entsprechende Ernen- nungsurkunde wurde ihm von Samtgemeindebürger- meister Jörg Warncke ausgehändigt. Samtgemeindebürgermeister Jörg Warncke hat Herrn Hebecker die Ernennungsurkunde überreicht Die Entscheidung zeigt, dass die Leistungen von Herrn Hebecker in den letzten 11 Jahren auch im Rat der Samtge- meinde sehr positiv aufgenommen wurden und so zu dieser einvernehmlichen Entscheidung geführt haben. Somit ist sichergestellt, dass in dieser sehr wichtigen Funktion auch zukünftig wieder ein qualifizierter Beamter tätig sein wird. Wir wünschen Herrn Hebecker viel Erfolg bei der Erfüllung seiner zukünftigen Aufgaben! Sprechstunden der Bürgermeister in der Samtgemeinde Samtgemeinde Lachendorf: Ahnsbeck: Ulrich Kaiser Samtgemeindebürgermeister Jörg Warncke Jederzeit nach vorheriger telefonischer Zu den allgemeinen Öffnungszeiten des Rat- Terminvereinbarung hauses. -

1469 Vol 43#5 Art 03.Indd
1469 The Canadian Mineralogist Vol. 43, pp. 1469-1487 (2005) BORATE MINERALS OF THE PENOBSQUIS AND MILLSTREAM DEPOSITS, SOUTHERN NEW BRUNSWICK, CANADA JOEL D. GRICE§, ROBERT A. GAULT AND JERRY VAN VELTHUIZEN† Research Division, Canadian Museum of Nature, P.O. Box 3443, Station D, Ottawa, Ontario K1P 6P4, Canada ABSTRACT The borate minerals found in two potash deposits, at Penobsquis and Millstream, Kings County, New Brunswick, are described in detail. These deposits are located in the Moncton Subbasin, which forms the eastern portion of the extensive Maritimes Basin. These marine evaporites consist of an early carbonate unit, followed by a sulfate, and fi nally, a salt unit. The borate assemblages occur in specifi c beds of halite and sylvite that were the last units to form in the evaporite sequence. Species identifi ed from drill-core sections include: boracite, brianroulstonite, chambersite, colemanite, congolite, danburite, hilgardite, howlite, hydroboracite, kurgantaite, penobsquisite, pringleite, ruitenbergite, strontioginorite, szaibélyite, trembathite, veatchite, volkovskite and walkerite. In addition, 41 non-borate species have been identifi ed, including magnesite, monohydrocalcite, sellaite, kieserite and fl uorite. The borate assemblages in the two deposits differ, and in each deposit, they vary stratigraphically. At Millstream, boracite is the most common borate in the sylvite + carnallite beds, with hilgardite in the lower halite strata. At Penobsquis, there is an upper unit of hilgardite + volkovskite + trembathite in halite and a lower unit of hydroboracite + volkov- skite + trembathite–congolite in halite–sylvite. At both deposits, values of the ratio of B isotopes [␦11B] range from 21.5 to 37.8‰ [21 analyses] and are consistent with a seawater source, without any need for a more exotic interpretation. -

Evolution of the Astonishing Naica Giant Crystals in Chihuahua, Mexico
minerals Review Evolution of the Astonishing Naica Giant Crystals in Chihuahua, Mexico Iván Jalil Antón Carreño-Márquez 1 , Isaí Castillo-Sandoval 2, Bernardo Enrique Pérez-Cázares 3, Luis Edmundo Fuentes-Cobas 2 , Hilda Esperanza Esparza-Ponce 2 , Esperanza Menéndez-Méndez 4, María Elena Fuentes-Montero 3 and María Elena Montero-Cabrera 2,* 1 Department of Engineering, Universidad La Salle Chihuahua, Chihuahua 31625, Mexico; [email protected] 2 Department of Environment and Energy, Centro de Investigación en Materiales Avanzados, Chihuahua 31136, Mexico; [email protected] (I.C.-S.); [email protected] (L.E.F.-C.); [email protected] (H.E.E.-P.) 3 Department of Computational Chemistry, Universidad Autónoma de Chihuahua, Chihuahua 31125, Mexico; [email protected] (B.E.P.-C.); [email protected] (M.E.F.-M.) 4 Department Physicochemical Assays, Instituto Eduardo Torroja de Ciencias de la Construcción, 28033 Madrid, Spain; [email protected] * Correspondence: [email protected] Abstract: Calcium sulfate (CaSO4) is one of the most common evaporites found in the earth’s crust. It can be found as four main variations: gypsum (CaSO4·2H2O), bassanite (CaSO4·0.5H2O), soluble Citation: Carreño-Márquez, I.J.A.; anhydrite, and insoluble anhydrite (CaSO4), being the key difference the hydration state of the Castillo-Sandoval, I.; Pérez-Cázares, sulfate mineral. Naica giant crystals’ growth starts from a supersaturated solution in a delicate B.E.; Fuentes-Cobas, L.E.; Esparza- thermodynamic balance close to equilibrium, where gypsum can form nanocrystals able to grow Ponce, H.E.; Menéndez-Méndez, E.; up to 11–12 m long. -

Flyer Radtour Wathlingen
Infos zur Tour Wissenswertes Region Celle WATHLINGEN Alte trifft neue Energie Landkreis Celle Torfabbau Entdecken Sie die Region Celle mit dem Rad! Der fl ussbe- Ländliche Idylle im Land gleitende Aller-Radweg oder die Thementouren im und um Celle Torf diente zum Heizen und Kochen der nickenden Pferde den Naturpark Südheide erwarten Sie. Das gut beschilderte Alte tri t neue Energie Die Bewohner von Großmoor und Adelheidsdorf gingen bis Radwegenetz der Region bietet unzählige Variationsmög- Wathlingen ca. 1964 noch ins Moor, um Torf zum Heizen und Kochen lichkeiten. Auf unserem Region-Celle-Navigator haben wir Ländliche Idylle im Land In einem beschaulichen Mosaik aus zu stechen. Die Torfschicht war bis zu 1,80 m tief. Durch die die schönsten Strecken für Sie zusammen gestellt: der nickenden Pferde Wäldern, Hecken, Feldern und Wiesen vorangegangene Trockenlegung konnten die Flächen begangen www.region-celle-navigator.de begegnen Ihnen viele Spuren der Energiegewinnung: werden, ohne zu versinken. Der Torf wurde Schicht um Schicht Blick zum Kaliberg © Blattwerker.de von Erdöl-Förderpumpen, die an nickende Pferde erinnern, mit einem scharfen, langen Spaten in kleine, rechteckige Der Landkreis Celle ist 2014 für über den Brennstoff Torf bis zu Windrädern und Solaranlagen. Ballen gestochen und zum Trocknen gestapelt. Weil der Torf Jahr 1910 mit der erfolgreichen Bohrung bis in 700 m Zieltiefe. sein radtouristisches Angebot vom Ein Blickfang auf dieser 35 km langen Tour ist der Kalimand- im großen Moor Richtung Ehlershausen nasser war, musste Nach der Stilllegung im Jahr 1996 wurde der Förderturm Land Niedersachsen ausgezeichnet scharo, wie die alte Kalihalde auch genannt wird. er vor dem Trocknen noch „gebacken“ werden. -
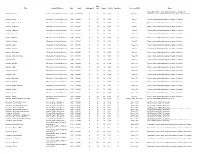
Um-Maps---G.Pdf
Map Title Author/Publisher Date Scale Catalogued Case Drawer Folder Condition Series or I.D.# Notes Topography, towns, roads, political boundaries for parts of Gabon - Libreville Service Géographique de L'Armée 1935 1:1,000,000 N 35 10 G1-A F One sheet Cameroon, Gabon, all of Equatorial Guinea, Sao Tomé & Principe Gambia - Jinnak Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 1 Towns, roads, political boundaries for parts of Gambia Gambia - N'Dungu Kebbe Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 2 Towns, roads, political boundaries for parts of Gambia Gambia - No Kunda Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 4 Towns, roads, political boundaries for parts of Gambia Gambia - Farafenni Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 5 Towns, roads, political boundaries for parts of Gambia Gambia - Kau-Ur Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 6 Towns, roads, political boundaries for parts of Gambia Gambia - Bulgurk Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 6 A Towns, roads, political boundaries for parts of Gambia Gambia - Kudang Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 7 Towns, roads, political boundaries for parts of Gambia Gambia - Fass Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 7 A Towns, roads, political boundaries for parts of Gambia Gambia - Kuntaur Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 8 Towns, roads, political -

179 Overprinting of Hydrothermal Regimes I N
179 OVERPRINTING OF HYDROTHERMAL REGIMES IN THE PALIMPINON GEOTHERMAL F I E L D , SOUTHERN NEGROS, PHILIPPINES T.M. L e a c h and I.Bogie Kingston Reynol ds Thom 1ardice Limited (KRTA) ABSTRACT major hydrothermal regimes are evident from the teration mineralogy in the Palimpinon Geothermal Field. A relict mineral zonation consisting potassic, advanced argill and propyl itic zones appears to have formed in response to the intrusion of a large in the western section of the field. A recent mineral zonation, that is interpreted t o have formed during the current geothermal system, is superimposed on the relict system and appears to be centered around the eastern portion of the field. The ict assemblages have many characteristics of a failed or barren porphyry copper system. The ict advanced ic mineralogy is interpreted to be of magmatic fluid origin and probably has not formed from the present geothermal regime with its Figure la: Well locations and cross section line predominant meteoric fluid component. The abundant Palimpinon Geothermal anhydrite being deposited in this geothermal system Field. Resistivity contours are shown is interpreted to have originated by redistribution in ohm-metres = 500 m) of anhydrite formed initially during the relict mag- matic hydrothermal system. Most Philippine systems are similar. INTRODUCT I0N Elevation An of the location and development of the Palimpinon field is given by Maunder et al. (1982). A general stratigraphy and subsurface geology derived from well geology is given in The youngest formation, the Cuernos Volcanics .) consists of an upper dacite unit (with a age of 14,000 years B.P.) and a lower clinopyroxene andesite. -

Assessment of the Molecular Structure of the Borate Mineral Boracite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 96 (2012) 946–951 Contents lists available at SciVerse ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa Assessment of the molecular structure of the borate mineral boracite Mg3B7O13Cl using vibrational spectroscopy ⇑ Ray L. Frost a, , Yunfei Xi a, Ricardo Scholz b a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, G.P.O. Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil highlights graphical abstract " Boracite is a magnesium borate mineral with formula: Mg3B7O13Cl. " The crystals belong to the orthorhombic – pyramidal crystal system. " The molecular structure of the mineral has been assessed. " Raman spectrum shows that some Cl anions have been replaced with OH units. article info abstract Article history: Boracite is a magnesium borate mineral with formula: Mg3B7O13Cl and occurs as blue green, colorless, Received 23 May 2012 gray, yellow to white crystals in the orthorhombic – pyramidal crystal system. An intense Raman band Received in revised form 2 July 2012 À1 at 1009 cm was assigned to the BO stretching vibration of the B7O13 units. Raman bands at 1121, Accepted 9 July 2012 1136, 1143 cmÀ1 are attributed to the in-plane bending vibrations of trigonal boron. Four sharp Raman Available online 13 August 2012 bands observed at 415, 494, 621 and 671 cmÀ1 are simply defined as trigonal and tetrahedral borate bending modes. The Raman spectrum clearly shows intense Raman bands at 3405 and 3494 cmÀ1, thus Keywords: indicating that some Cl anions have been replaced with OH units.