Dissertation / Doctoral Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gastrointestinal Defense Mechanisms
REVIEW CURRENT OPINION Gastrointestinal defense mechanisms Hyder Said a,b and Jonathan D. Kaunitzb,c Purpose of review To summarize and illuminate the recent findings regarding gastroduodenal mucosal defense mechanisms and the specific biomolecules involved in regulating this process, such as glucagon-like peptides (GLPs). Recent findings There has been a growing interest in luminal nutrient chemosensing and its physiological effects throughout the digestive system. From the ingestion of food in the oral cavity to the processing and absorption of nutrients in the intestines, nutrient chemosensing receptors signal the production and release of numerous bioactive peptides from enteroendocrine cells, such as the proglucagon-derived peptides. There has been a major emphasis on two proglucagon-derived peptides, namely GLP-1 and GLP-2, due to their apparent beneficial effect on gut structure, function, and on metabolic processes. As an incretin, GLP-1 not only enhances the effect and release of insulin on pancreatic bcells but also has been implicated in having trophic effects on the intestinal epithelium. In addition, GLP-2, the other major proglucagon-derived peptide, has potent intestinotrophic effects, such as increasing the rate of mucosal stem cell proliferation, mucosal blood flow, and fluid absorption, as well as augmenting the rate of duodenal bicarbonate secretion to improve gastric mucosal health and longevity. Summary Understanding the mechanisms underlying nutrient chemosensing and how it relates to GLP release can further elucidate how the gut functions in response to cellular changes and disturbances. Furthermore, a more in-depth comprehension of GLP release and its tissue-specific effects will help improve the utility of GLP-1 and GLP-2 receptor agonists in clinical settings. -

Activation Mechanism of the G Protein-Coupled Sweet Receptor Heterodimer with Sweeteners and Allosteric Agonists
Supporting Information Activation mechanism of the G protein-coupled sweet receptor heterodimer with sweeteners and allosteric agonists Soo-Kyung Kim, *† Yalu Chen, † Ravinder Abrol, †, ‡ William A. Goddard III,*† and Brian Guthrie§ †Materials and Process Simulation Center (MC 139-74), California Institute of Technology, 1200 E. California Blvd., Pasadena, CA 91125; ‡Current address, Departments of Chemistry and Biochemistry, California State University, Northridge, CA 91330; §Cargill Global Food Research, 2301 Crosby Road, Wayzata, MN 55391 * CORRESPONDING AUTHOR Prof. William A. Goddard III California Institute of Technology MC 139-74, 1200 E. California Blvd., Pasadena, CA 91125; phone: 1-626-395-2731, e-mail: [email protected] Dr. Soo-Kyung Kim phone: 1-626-395-2724, e-mail: [email protected] 1 RESULTS Structures for Allosteric ligand bound at the TMD of all three TAS1Rs As described in the METHODS section, the DarwinDock procedure (1) involves sampling ~50,000 poses for each of ~10 diverse ligand conformations from which, we select finally two energetically favorable binding poses based on two scoring methods: UCav E: unified cavity energy for which we consider that interactions of the best 100 poses with the union of all residues involve in their separate binding sites (providing a uniform comparison) BE: snap binding energy considering all interactions of ligand with protein As a first validation of the predicted structures for the 7 helix TMD, we used DarwinDock to predict the binding site for the allosteric ligands to each TAS1R TMD in Table S10. Here we find, S819 [1-((1H-pyrrol-2-yl)methyl)-3-(4-isopropoxyphenyl)thiourea] is a sweet compound that interacts with the TAS1R2 TMD.(2) and Lactisole is a competitive inhibitor of the sweet taste receptor that binds to TAS1R3 TMD.(3, 4) These structures were further relaxed through annealing. -

The Association of Bovine T1R Family of Receptors Polymorphisms with Cattle Growth Traits ⇑ C.L
Research in Veterinary Science xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Research in Veterinary Science journal homepage: www.elsevier.com/locate/rvsc The association of bovine T1R family of receptors polymorphisms with cattle growth traits ⇑ C.L. Zhang a, J. Yuan a, Q. Wang a, Y.H. Wang a, X.T. Fang a, C.Z. Lei b, D.Y. Yang c, H. Chen a, a Institute of Cellular and Molecular Biology, Xuzhou Normal University, Xuzhou, Jiangsu, PR China b College of Animal Science and Technology, Northwest Agriculture and Forestry University, Shaanxi Key Laboratory of Molecular Biology for Agriculture, Yangling, Shaanxi, PR China c College of Life Science, Dezhou University, Dezhou, Shandong 253023, PR China article info abstract Article history: The three members of the T1R class of taste-specific G protein-coupled receptors have been proven to Received 12 August 2011 function in combination with heterodimeric sweet and umami taste receptors in many mammals that Accepted 20 January 2012 affect food intake. This may in turn affect growth traits of livestock. We performed a comprehensive eval- Available online xxxx uation of single-nucleotide polymorphisms (SNPs) in the bovine TAS1R gene family, which encodes receptors for umami and sweet tastes. Complete DNA sequences of TAS1R1-, TAS1R2-, and TAS1R3-cod- Keywords: ing regions, obtained from 436 unrelated female cattle, representing three breeds (Qinchuan, Jiaxian Red, Taste receptors Luxi), revealed substantial coding and noncoding diversity. A total of nine SNPs in the TAS1R1 gene were SNP identified, among which seven SNPs were in the coding region, and two SNPs were in the introns. -

(Rs12033832) and Sweet Taste Perception Amongst Malay Obese and Non- Obese Subjects Ahmad Riduan Bahauddin1, Nazamid Shaari2, Zalilah Mohd Shariff3, Roselina Karim2
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346) ORIGINAL ARTICLE Association Between TAS1R2 Gene Polymorphism (rs12033832) and Sweet Taste Perception Amongst Malay Obese and Non- obese Subjects Ahmad Riduan Bahauddin1, Nazamid Shaari2, Zalilah Mohd Shariff3, Roselina Karim2 1 Faculty of Food Science and Nutrition, Universiti Malaysia Sabah, Jln. UMS, 88400 Kota Kinabalu, Sabah, Malaysia 2 Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43000, Serdang, Selangor, Malaysia 3 Department of Nutrition and Dietetics, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43000 Serdang, Selangor, Malaysia ABSTRACT Introduction: A growing evidence supported that variation of sweet taste perception, mediated by TAS1Rs gene variants could lead to excess sweetened food and beverages intake and also obesity. However, obesity development may also alter individuals' taste sensitivity and perception. Thus, it is best to further investigate whether or not the individuals' sweet taste sensitivity and acceptance are associated with variation in TAS1R2 gene and Body Mass Index (BMI) status. Methods: This comparison cross sectional study comprised of 88 obese and 92 non-obese subjects aged 20-45. All the subjects were genotyped for TAS1R2 gene variant at rs12033832 using polymerase chain reaction – restriction fragment length polymorphism (PCR-RFLP). Suprathreshold sensitivity for sweet taste was assessed using general Labeled Magnitude Scales. Intensity rating and hedonic test were carried out on 2 food samples (tea drink and rose flavoured agar) to examine subject's intensity rating and liking at different sugar contents. Results: Our re- sults showed that rs12033832 of TAS1R2 gene is associated with sweet taste perception among obese and non-obese subjects. -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

Methods to Identify Tas2r Modulators Verfahren Zur Identifizierung Von Tas2r Modulatoren Procédé D’Identification De Modulateurs Tas2r
(19) TZZ _¥¥ _T (11) EP 2 137 322 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12Q 1/68 (2006.01) A23L 2/52 (2006.01) 27.02.2013 Bulletin 2013/09 A23G 4/00 (2006.01) C07C 53/134 (2006.01) (21) Application number: 08714784.9 (86) International application number: PCT/CH2008/000134 (22) Date of filing: 27.03.2008 (87) International publication number: WO 2008/119195 (09.10.2008 Gazette 2008/41) (54) METHODS TO IDENTIFY TAS2R MODULATORS VERFAHREN ZUR IDENTIFIZIERUNG VON TAS2R MODULATOREN PROCÉDÉ D’IDENTIFICATION DE MODULATEURS TAS2R (84) Designated Contracting States: • BEHRENS MAIK ET AL: "Members of RTP and AT BE BG CH CY CZ DE DK EE ES FI FR GB GR REEP gene families influence functional bitter HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT taste receptor expression" JOURNAL OF RO SE SI SK TR BIOLOGICAL CHEMISTRY, vol. 281, no. 29, July 2006(2006-07), pages 20650-20659, XP002494217 (30) Priority: 30.03.2007 US 909143 P ISSN: 0021-9258 30.07.2007 US 962549 P • KUHN CHRISTINA ET AL: "Bitter taste receptors for saccharin and acesulfame K" JOURNAL OF (43) Date of publication of application: NEUROSCIENCE, vol. 24, no. 45, 10 November 30.12.2009 Bulletin 2009/53 2004 (2004-11-10), pages 10260-10265, XP002494218 ISSN: 0270-6474 (73) Proprietor: Givaudan SA • BUFE BERND ET AL: "The human TAS2R16 1214 Vernier (CH) receptor mediates bitter taste in response to beta- glucopyranosides" NATURE GENETICS, (72) Inventors: NATURE PUBLISHING GROUP, NEW YORK, US, • BRUNE, Nicole, Erna, Irene vol. -

Human Pluripotent Stem Cell-Derived Ectomesenchymal Stromal Cells Promote More Robust Functional Recovery Than Umbilical Cord-De
Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic- ischaemic brain damage Jiawei Huang1,3*, Kin Pong U1,3*, Fuyuan Yang1, Zeyuan Ji1, Jiacheng Lin1,3, Zhihui Weng1, Lai Ling Tsang1,3, Tobias D Merson5, Ye Chun Ruan6, Chao Wan1,3, Gang Li2, Xiaohua Jiang1,3,4 1School of Biomedical Sciences, 2Department of Orthopaedics and Traumatology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, PR China. 3School of Biomedical Sciences Core Laboratory, Shenzhen Research Institute, The Chinese University of Hong Kong, Shenzhen, PR China. 4Sichuan University – The Chinese University of Hong Kong Joint Laboratory for Reproductive Medicine, West China Second University Hospital, Sichuan University, Chengdu 610041, Sichuan, China. 5Australian Regenerative Medicine Institute, Monash University, Clayton, VIC, Australia. 6Department of Biomedical Engineering, Faculty of Engineering, The Hong Kong Polytechnic University, Hong Kong, China. Running Title: Human ectomesenchymal stromal cells promote functional recovery in a rat HIE model *Corresponding author: Prof. Xiaohua JIANG, Email: [email protected] Address: Room 409A, Lo Kwee Seong Integrated Biomedical Sciences Building, Area 39, The Chinese University of Hong Kong, Shatin. Keywords: HIE, ectomesenchymal stromal cells, brain damage, regeneration, paracrine, ERK Abstract: Aims: Hypoxic-ischaemic encephalopathy (HIE) is one of the most serious complications in neonates and infants. Mesenchymal stromal cell (MSC)-based therapy is emerging as a promising treatment avenue for HIE. However, despite its enormous potential, the clinical application of MSCs is limited by cell heterogeneity, low isolation efficiency and unpredictable effectiveness. In this study, we examined the therapeutic effects and underlying mechanisms of human pluripotent stem cell-derived ectomesenchymal stromal cells (hPSC-EMSCs) in a rat model of HIE. -
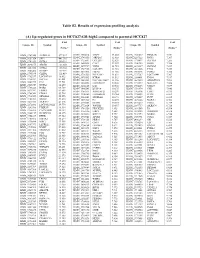
(A) Up-Regulated Genes in HCC827-GR-High2 Compared to Parental HCC827
Table S2. Results of expression profiling analysis (A) Up-regulated genes in HCC827-GR-high2 compared to parental HCC827 Fold Fold Fold Unique ID Symbol Unique ID Symbol Unique ID Symbol change* change* change* ILMN_1709348 ALDH1A1 577.587 ILMN_2310814 MAPT 13.003 ILMN_1741017 PIP4K2B 7.331 ILMN_1651354 SPP1 441.316 ILMN_1748650 MRPL45 12.988 ILMN_3237623 RNY1 7.297 ILMN_1701831 GSTA1 260.591 ILMN_1755897 UGT2B7 12.629 ILMN_1734897 SLC4A4 7.285 ILMN_1658835 CAV2 12.357 ILMN_1746359 RERG 7.280 ILMN_2094875 ABCB1 183.050 ILMN_1678939 VNN2 11.935 ILMN_1671337 SLC2A5 7.257 ILMN_3251540 GSTA2 145.982 ILMN_1729905 GAL3ST1 11.910 ILMN_1691606 LYG2 7.254 ILMN_2062468 IGFBP7 127.721 ILMN_1672536 FBLN1 11.716 ILMN_1785646 PMP22 7.246 ILMN_1795190 CLDN2 111.439 ILMN_1796339 PLEKHA2 11.631 ILMN_1737387 LOC728441 7.207 ILMN_1782937 LOC647169 98.612 ILMN_1676563 HTRA1 11.592 ILMN_1684401 FMO1 7.117 ILMN_1754247 SLC3A1 81.001 ILMN_3263423 LOC100129027 11.346 ILMN_1687035 ADAMTSL4 7.098 ILMN_1662795 CA2 79.581 ILMN_1694898 LOC653857 10.906 ILMN_2153572 MAGEA3 7.086 ILMN_2168747 GSTA2 66.250 ILMN_2404625 LAT 10.560 ILMN_1784283 USH1C 7.079 ILMN_1764228 DAB2 64.709 ILMN_1666546 DUSP14 10.375 ILMN_1731374 CPE 7.046 ILMN_1675797 EPDR1 63.605 ILMN_1764571 ARHGAP23 10.299 ILMN_1765446 EMP3 6.933 ILMN_1708341 PDZK1 59.714 ILMN_3200140 LOC645638 10.284 ILMN_1754002 IL1F8 6.863 ILMN_1713529 SEMA6A 52.575 ILMN_3244343 SNORA21 10.171 ILMN_1878007 FUT9 6.835 ILMN_1708391 NR1H4 43.218 ILMN_1671489 PC 10.075 ILMN_1699208 NAP1L1 6.763 ILMN_2412336 AKR1C2 42.826 ILMN_2404688 -

The Potential Druggability of Chemosensory G Protein-Coupled Receptors
International Journal of Molecular Sciences Review Beyond the Flavour: The Potential Druggability of Chemosensory G Protein-Coupled Receptors Antonella Di Pizio * , Maik Behrens and Dietmar Krautwurst Leibniz-Institute for Food Systems Biology at the Technical University of Munich, Freising, 85354, Germany; [email protected] (M.B.); [email protected] (D.K.) * Correspondence: [email protected]; Tel.: +49-8161-71-2904; Fax: +49-8161-71-2970 Received: 13 February 2019; Accepted: 12 March 2019; Published: 20 March 2019 Abstract: G protein-coupled receptors (GPCRs) belong to the largest class of drug targets. Approximately half of the members of the human GPCR superfamily are chemosensory receptors, including odorant receptors (ORs), trace amine-associated receptors (TAARs), bitter taste receptors (TAS2Rs), sweet and umami taste receptors (TAS1Rs). Interestingly, these chemosensory GPCRs (csGPCRs) are expressed in several tissues of the body where they are supposed to play a role in biological functions other than chemosensation. Despite their abundance and physiological/pathological relevance, the druggability of csGPCRs has been suggested but not fully characterized. Here, we aim to explore the potential of targeting csGPCRs to treat diseases by reviewing the current knowledge of csGPCRs expressed throughout the body and by analysing the chemical space and the drug-likeness of flavour molecules. Keywords: smell; taste; flavour molecules; drugs; chemosensory receptors; ecnomotopic expression 1. Introduction Thirty-five percent of approved drugs act by modulating G protein-coupled receptors (GPCRs) [1,2]. GPCRs, also named 7-transmembrane (7TM) receptors, based on their canonical structure, are the largest family of membrane receptors in the human genome. -

Strand Breaks for P53 Exon 6 and 8 Among Different Time Course of Folate Depletion Or Repletion in the Rectosigmoid Mucosa
SUPPLEMENTAL FIGURE COLON p53 EXONIC STRAND BREAKS DURING FOLATE DEPLETION-REPLETION INTERVENTION Supplemental Figure Legend Strand breaks for p53 exon 6 and 8 among different time course of folate depletion or repletion in the rectosigmoid mucosa. The input of DNA was controlled by GAPDH. The data is shown as ΔCt after normalized to GAPDH. The higher ΔCt the more strand breaks. The P value is shown in the figure. SUPPLEMENT S1 Genes that were significantly UPREGULATED after folate intervention (by unadjusted paired t-test), list is sorted by P value Gene Symbol Nucleotide P VALUE Description OLFM4 NM_006418 0.0000 Homo sapiens differentially expressed in hematopoietic lineages (GW112) mRNA. FMR1NB NM_152578 0.0000 Homo sapiens hypothetical protein FLJ25736 (FLJ25736) mRNA. IFI6 NM_002038 0.0001 Homo sapiens interferon alpha-inducible protein (clone IFI-6-16) (G1P3) transcript variant 1 mRNA. Homo sapiens UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 15 GALNTL5 NM_145292 0.0001 (GALNT15) mRNA. STIM2 NM_020860 0.0001 Homo sapiens stromal interaction molecule 2 (STIM2) mRNA. ZNF645 NM_152577 0.0002 Homo sapiens hypothetical protein FLJ25735 (FLJ25735) mRNA. ATP12A NM_001676 0.0002 Homo sapiens ATPase H+/K+ transporting nongastric alpha polypeptide (ATP12A) mRNA. U1SNRNPBP NM_007020 0.0003 Homo sapiens U1-snRNP binding protein homolog (U1SNRNPBP) transcript variant 1 mRNA. RNF125 NM_017831 0.0004 Homo sapiens ring finger protein 125 (RNF125) mRNA. FMNL1 NM_005892 0.0004 Homo sapiens formin-like (FMNL) mRNA. ISG15 NM_005101 0.0005 Homo sapiens interferon alpha-inducible protein (clone IFI-15K) (G1P2) mRNA. SLC6A14 NM_007231 0.0005 Homo sapiens solute carrier family 6 (neurotransmitter transporter) member 14 (SLC6A14) mRNA.