Carbonic Acids and Heterofunctional Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
CH. 13 Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution Electrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. Recall: R3 R4 E Y E Y C C + E Y R1 C C R4 R1 C C R4 − R2 R1 δ+ δ R2 R3 R2 R3 In aromatic rings, however, we see substitution of one of the benzene ring hydrogens for an electrophile. H E + E Y + Y H δ+ δ− The mechanism is the same regardless of the electrophile. It involves two steps: (1) Addition of the electrophile to form a high-energy carbocation. (2) Elimination of the proton to restore the aromatic ring system. H H H H slow E Y + Y step 1 + E δ+ δ− high energy arenium ion H H step 2 fast E E + Y + Y H The first step is the slow step since the aromaticity of the benzene ring system is destroyed on formation of the arenium ion intermediate. This is a high energy species but it is stabilized by resonance with the remaining two double bonds. The second step is very fast since it restores the aromatic stabilization. 1 CH. 13 H H H H H H E E E There are five electrophilic aromatic substitution reactions that we will study. (1) Nitration H NO2 H2SO4 + HNO3 (2) Sulfonation H SO3H + H2SO4 (3) Halogenation with bromine or chlorine H X FeX3 X = Br, Cl + X2 (4) Friedel-Crafts Alkylation H R AlX + RX 3 (5) Friedel-Crafts Acylation O H O C AlX3 R + Cl C R 2 CH. -
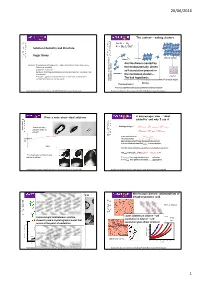
Roger Davey + Critical Nucleus Dimers Are the Dimers Created by Content: Studying and Defining Solute- Solute Interactions in the Liquid Phase
20/06/2016 The context – making clusters A + A A 2 K = [A ] / [A] 2 Solution Chemistry and Structure 2 Roger Davey + Critical nucleus dimers Are the dimers created by Content: Studying and defining solute- solute interactions in the liquid phase. Techniques available. thermodynamically driven Speciation in solution. self association present in Do these (thermodynamic features have) an impact on nucleation rate tetramers or outcome? monomers the nucleation clusters – Examples – glycine/saccharin/benzoic acid/tetrolic acid/mandelic crystal acid/paminobenzoic acid/co-crystals. The link hypothesis . Equilibria in supersaturated homogeneous solution Formation and Growth of critical nuclei Kinetics Thermodynamics Are these equilibria and quasi equilibria in anyway related? Nucleation Summer School June 20-24 th 2016 University of Strathclyde Nucleation Summer School June 20-24 th 2016 University of Strathclyde First a note about ideal solutions A macroscopic view - ‘ideal solubility’ and why I use it. Enthalpy changes: The usual way: ∆Hsolution = ∆H sublimation + ∆H solvation dissolve solid in ∆H = ∆H + ∆H solvent solution fusion mixing In an ideal solution ∆H mixing = 0. compositi This means that (Es-s + Esolv-solv )/2 = Es-solv on Real solutions don’t have this exact balance and in a non-ideal solution ∆H mixing is thus non zero. time And the ideal solubility is given by a very familiar equation: ln(xideal )={ΔHF(1/T m-1/T)+ ΔCp[T m/T – ln(T m/T)-1]}/R The ideal way: melt solid and mix with solvent. If x > xideal then negative deviation …….solvation If x < xideal then positive deviation………aggregation. Nucleation Summer School June 20-24 th 2016 University of Strathclyde Nucleation Summer School June 20-24 th 2016 University of Strathclyde Macroscopic picture: polymorphism of Macroscopic picture: saccharin dihydroxybenzoic acid. -
Chemical Specificity in Short-Chain Fatty Acids and Their Analogues in Increasing Osmotic Fragility in Rat Erythrocytes in Vitro
Chemical specificity in short-chain fatty acids and their analogues in increasing osmotic fragility in rat erythrocytes in Title vitro. Author(s) Mineo, Hitoshi; Hara, Hiroshi Biochimica et Biophysica Acta (BBA) - Biomembranes, 1768(6), 1448-1453 Citation https://doi.org/10.1016/j.bbamem.2007.02.008 Issue Date 2007-06 Doc URL http://hdl.handle.net/2115/28208 Type article (author version) File Information BBA1768-6.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 1 Chemical specificity in short-chain fatty acids and their analogues in increasing osmotic 2 fragility in rat erythrocytes in vitro 3 4 Hitoshi Mineo, Hiroshi Hara* 5 6 Division of Applied Bioscience, Graduate School of Agriculture, Hokkaido University, 7 Hokkaido 060-8589, Japan 8 9 10 *Corresponding author. 11 Hiroshi HARA Ph.D. 12 Division of Applied Bioscience, 13 Graduate School of Agriculture, 14 Hokkaido University, 15 Kita-9, Nishi-9, Sapporo, 16 Hokkaido 060-8589, 17 Tel.: +81-11-706-3352; 18 fax: +81-11-706-2504. 19 E-mail address: [email protected] 20 21 22 23 24 25 2 1 Abstract 2 3 We examined the role of the chemical specificity of short-chain fatty acids 4 (SCFAs) and their derivatives in increasing osmotic fragility (OF) in rat red blood cells 5 (RBCs). Except for formic acid, normal SCFAs with 2 to 8 carbons increased the OF in 6 rat RBCs with increasing number of hydrocarbons in a dose-dependent manner. 7 Replacement of the carboxylic group with sulfonic group inhibited, but did not abolish, 8 the SCFA-mediated increase in OF. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing -

United States Patent (19) 11 4,027,037 Siegle Et Al
United States Patent (19) 11 4,027,037 Siegle et al. (45) May 31, 1977 (54) N-SUBSTITUTED B-AMINOCROTONIC 57) ABSTRACT ACID ESTERS N-substituted (3-aminocrotonic acid esters of the for mula (75) Inventors: Peter Siegle, Cologne; Klaus Sasse, Schildgen; Peter Rössler, Cologne, all of Germany CH. COOR: (I) RC=CCN H (73) Assignee: Bayer Aktiengesellschaft, R11 Leverkusen, Germany in which (22 Filed: Mar. 12, 1975 R is hydrogen, or alkyl or alkenyl with up to 4 car bon atoms, (21) Appl. No.: 557,698 R’ is straight-chain or branched alkyl or alkenyl with up to 9 carbon atoms, optionally interrupted by O 30 Foreign Application Priority Data or S, and optionally substituted by halogen, phe Mar. 26, 1974 Germany .......................... 2414456 noxy, dimethylamino, cyclic alkyl or alkenyl with up to 8 carbon atoms which are optionally substi (52) U.S. C. ......................... 424/314; 260/239 BE; tuted by halogen, dimethylamino, or alkyl, alkoxy 260/239 BF; 260/247.2 B; 260/268 R; or alkylthio with up to 4 carbon atoms, phenyl 260/293.88; 260/326.43; 260/340.5; which is optionally substituted by halogen, dimeth 260/347.4; 260/468 G; 260/468 J; 260/470; ylamino, phenoxy, or alkyl, alkenyl, acyl, alkoxy, 260/471 A; 260/479 S; 260/481 R; 260/482 alkenoxy or alkynoxy containing up to 4 carbon R; 424/244; 424/2 48.52; 424/250; 424/267; atoms, or a 5- or 6-membered heterocyclic ring 424/274; 424/282; 424/285; 424/299; containing at least one O, S or N hetero-atom and 424/309; 424/248.55 optionally substituted by halogen, alkyl or alkoxy containing up to 4 carbon atoms or R is further (51 int. -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Comparative Responses of Rice (Oryza Sativa) Straw to Urea Supplementation and Urea Treatment
COMPARATIVE RESPONSES OF RICE (ORYZA SATIVA) STRAW TO UREA SUPPLEMENTATION AND UREA TREATMENT M. N. A. Kumar1, K. Sundareshan, E. G. Jagannath S. R. Sampath and P. T. Doyle2 Southern Regional Station, National Dairy Research Institute, Bangalore - 560030, India Summary Twenty five 75% Holstein Friesian cross bred bullocks fed rice straw (Oryza saliva) of long form, were fed with the following five treatments. 1. Rice straw, untreated (RS) 2. RS + water (1:1), stored for 24 hours (WRS) 3. RS (100 kg) + urea solution (4 kg urea/100 litre water) and dried (USRS) 4. RS (100 kg) + urea solution (as in 3) stored in wet condition for 24 hours (UWRS) 5. RS (100 kg) + urea solution (as in 3) stored in pit for 21 days (UTRS). Potential digestibility of treatments of RS was evaluated by monitoring (in vitro) Simulating Rumen like Fermentation (SRLF). The results indicated that Dry Matter Intake (DMI), digestibility of nutrients, N utilization were of the order UTRS > UWRS > USRS > WRS and RS (p < 0.05 to P < 0.01). SRLF index was high (255.84) for UTRS and least (145.58) for USRS. It was interm ediary (199.66) for UWRS. The acetyl content (AC) of UTRS with higher hemicellulose (HCE) dig estibility (80.8%) was low compared to UWRS, USRS, RS and WRS. The acetate content was of the order UTRS < UWRS < USRS < WRS and RS thereby indicating that reduction in acetyl con tent was an index of positive response of urea-treatment of RS. In addition, the ratio of HCE/AC in faeces of UTRS was 0.87 as against the ratios (2.26-2.48) observed in other treatments recording reduction jn AC due to urea-treatinent. -

Molecular Structure of Wlbb, a Bacterial N-Acetyltransferase Involved in the Biosynthesis †,‡ of 2,3-Diacetamido-2,3-Dideoxy-D-Mannuronic Acid James B
4644 Biochemistry 2010, 49, 4644–4653 DOI: 10.1021/bi1005738 Molecular Structure of WlbB, a Bacterial N-Acetyltransferase Involved in the Biosynthesis †,‡ of 2,3-Diacetamido-2,3-dideoxy-D-mannuronic Acid James B. Thoden and Hazel M. Holden* Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706 Received April 14, 2010; Revised Manuscript Received April 29, 2010 ABSTRACT: The pathogenic bacteria Pseudomonas aeruginosa and Bordetella pertussis contain in their outer membranes the rare sugar 2,3-diacetamido-2,3-dideoxy-D-mannuronic acid. Five enzymes are required for the biosynthesis of this sugar starting from UDP-N-acetylglucosamine. One of these, referred to as WlbB, is an N-acetyltransferase that converts UDP-2-acetamido-3-amino-2,3-dideoxy-D-glucuronic acid (UDP- GlcNAc3NA) to UDP-2,3-diacetamido-2,3-dideoxy-D-glucuronic acid (UDP-GlcNAc3NAcA). Here we report the three-dimensional structure of WlbB from Bordetella petrii. For this analysis, two ternary structures were determined to 1.43 A˚resolution: one in which the protein was complexed with acetyl-CoA and UDP and the second in which the protein contained bound CoA and UDP-GlcNAc3NA. WlbB adopts a trimeric quaternary structure and belongs to the LβH superfamily of N-acyltransferases. Each subunit contains 27 β-strands, 23 of which form the canonical left-handed β-helix. There are only two hydrogen bonds that occur between the protein and the GlcNAc3NA moiety, one between Oδ1 of Asn 84 and the sugar C-30 amino group and the second between the backbone amide group of Arg 94 and the sugar C-50 carboxylate. -

215-216 HH W12-Notes-Ch 15
Chem 215 F12 Notes Notes – Dr. Masato Koreeda - Page 1 of 17. Date: October 5, 2012 Chapter 15: Carboxylic Acids and Their Derivatives and 21.3 B, C/21.5 A “Acyl-Transfer Reactions” I. Introduction Examples: note: R could be "H" R Z R O H R O R' ester O carboxylic acid O O an acyl group bonded to R X R S acid halide* R' an electronegative atom (Z) thioester O X = halogen O R' R, R', R": alkyl, alkenyl, alkynyl, R O R' R N or aryl group R" amide O O O acid anhydride one of or both of R' and R" * acid halides could be "H" R F R Cl R Br R I O O O O acid fluoride acid chloride acid bromide acid iodide R Z sp2 hybridized; trigonal planar making it relatively "uncrowded" O The electronegative O atom polarizes the C=O group, making the C=O carbon "electrophilic." Resonance contribution by Z δ * R Z R Z R Z R Z C C C C O O O δ O hybrid structure The basicity and size of Z determine how much this resonance structure contributes to the hybrid. * The more basic Z is, the more it donates its electron pair, and the more resonance structure * contributes to the hybrid. similar basicity O R' Cl OH OR' NR'R" Trends in basicity: O weakest increasing basiciy strongest base base Check the pKa values of the conjugate acids of these bases. Chem 215 F12 Notes Notes –Dr. Masato Koreeda - Page 2 of 17. -

(12) United States Patent (10) Patent No.: US 8,623.422 B2 Hansen Et Al
USOO8623422B2 (12) United States Patent (10) Patent No.: US 8,623.422 B2 Hansen et al. (45) Date of Patent: *Jan. 7, 2014 (54) COMBINATION TREATMENT WITH 5,668,161 A 9/1997 Talley et al. STRONTUM FOR THE PROPHYLAXIS 5,681,842 A 10, 1997 Dellaria et al. AND/OR TREATMENT OF CARTILAGE 5,686.460 A 11/1997 Nicolai et al. AND/OR BONE CONDITIONS 5,686,470 A 11/1997 Weier et al. 5,696,431 A 12/1997 Giannopoulos et al. 5,707,980 A 1/1998 Knutson (75) Inventors: Christian Hansen, Vedback (DK); 5,719,163 A 2f1998 Norman et al. Henrik Nilsson, Copenhagen (DK); 5,750,558 A 5/1998 Brooks et al. Stephan Christgau, Gentofte (DK) 5,753,688 A 5/1998 Talley et al. 5,756,530 A 5/1998 Lee et al. (73) Assignee: Osteologix A/S, Copenhagen (DK) 5,756,531 A 5/1998 Brooks et al. 5,760,068 A 6/1998 Talley et al. (*) Notice: Subject to any disclaimer, the term of this 5,776,967 A 7/1998 Kreft et al. patent is extended or adjusted under 35 5,776,984. A 7/1998 Dellaria et al. U.S.C. 154(b) by 558 days. 5,783,597 A 7/1998 Beers et al. This patent is Subject to a terminal dis 5,807,873 A 9, 1998 Nicolai et al. claimer. 5,824,699 A 10, 1998 Kreft et al. 5,830,911 A 11/1998 Failli et al. 5,840,924 A 11/1998 Desmond et al. -

Carboxylic Acids
13 Carboxylic Acids The active ingredients in these two nonprescription pain relievers are derivatives of arylpropanoic acids. See Chemical Connections 13A, “From Willow Bark to Aspirin and Beyond.” Inset: A model of (S)-ibuprofen. (Charles D. Winters) KEY QUESTIONS 13.1 What Are Carboxylic Acids? HOW TO 13.2 How Are Carboxylic Acids Named? 13.1 How to Predict the Product of a Fischer 13.3 What Are the Physical Properties of Esterification Carboxylic Acids? 13.2 How to Predict the Product of a B-Decarboxylation 13.4 What Are the Acid–Base Properties of Reaction Carboxylic Acids? 13.5 How Are Carboxyl Groups Reduced? CHEMICAL CONNECTIONS 13.6 What Is Fischer Esterification? 13A From Willow Bark to Aspirin and Beyond 13.7 What Are Acid Chlorides? 13B Esters as Flavoring Agents 13.8 What Is Decarboxylation? 13C Ketone Bodies and Diabetes CARBOXYLIC ACIDS ARE another class of organic compounds containing the carbonyl group. Their occurrence in nature is widespread, and they are important components of foodstuffs such as vinegar, butter, and vegetable oils. The most important chemical property of carboxylic acids is their acidity. Furthermore, carboxylic acids form numerous important derivatives, including es- ters, amides, anhydrides, and acid halides. In this chapter, we study carboxylic acids themselves; in Chapters 14 and 15, we study their derivatives. 457 458 CHAPTER 13 Carboxylic Acids 13.1 What Are Carboxylic Acids? Carboxyl group A J COOH The functional group of a carboxylic acid is a carboxyl group, so named because it is made group. up of a carbonyl group and a hydroxyl group (Section 1.7D). -

Chem 215 F11 Notes – Dr. Masato Koreeda - Page 1 of 14
Chem 215 F11 Notes – Dr. Masato Koreeda - Page 1 of 14. Date: September 30, 2011 Chapter 15: Carboxylic Acids and Their Derivatives – Acyl Transfer Reactions I. Introduction Examples: note: R could be "H" R Z R O H R O R' ester O carboxylic acid O O an acyl group bonded to R X R S acid halide* R' an electronegative atom (Z) thioester O X = halogen O R' R, R', R": alkyl, alkenyl, alkynyl, R O R' R N or aryl group R" amide O O O acid anhydride one of or both of R' and R" * acid halides could be "H" R F R Cl R Br R I O O O O acid fluoride acid chloride acid bromide acid iodide R Z sp2 hybridized; trigonal planar making it relatively "uncrowded" O The electronegative O atom polarizes the C=O group, making the C=O carbon "electrophilic." Resonance contribution by Z δ * R Z R Z R Z R Z C C C C O O O δ O hybrid structure The basicity and size of Z determine how much this resonance structure contributes to the hybrid. * The more basic Z is, the more it donates its electron pair, and the more resonance structure * contributes to the hybrid. similar basicity O R' Cl OH OR' NR'R" Trends in basicity: O weakest increasing basiciy strongest base base Check the pKa values of the conjugate acids of these bases. Chem 215 F11 Notes –Dr. Masato Koreeda - Page 2 of 14. Date: September 30, 2011 Relative stabilities of carboxylic acid derivatives against nucleophiles R Z As the basicity of Z increases, the stability of increases because of added resonance stabilization.