Downloaded 9/24/2021 4:45:01 AM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis of 1,2,4 Oxadiazol-5-Imine, 1,2,4-Triazol-3-Imine and Derivatives: a Substituted Cyanamide-Based Strategy for Heterocycle Synthesis
Synthesis of 1,2,4 oxadiazol-5-imine, 1,2,4-triazol-3-imine and derivatives: A Substituted Cyanamide-based Strategy for Heterocycle Synthesis Shreesha V. Bhat A thesis submitted in partial fulfilment of the requirements of the University of Lincoln for the degree of Doctor of Philosophy June 2017 Statement of Originality “I, Shreesha V. Bhat, hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been published or accepted for the award of any other degree or diploma at University of Lincoln or any other educational institution, except where references have been made in the thesis. Any contribution made to the research by others, with whom I have worked at the University of Lincoln or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.” (Shreesha V. Bhat) ii | P a g e Abstract Considering the importance of nitrogen-rich heterocycles in drug discovery, a novel strategy towards heterocycle synthesis was envisioned using cyanamide chemistry. Synthesis which involve mild conditions, avoids multi-step sequence and non-toxic reagents are desirable for generation of large combinatorial libraries of drug molecules. We envisaged that the NCN linkage of the cyanamide as well as the concomitant use of the nucleo-and electrophilic centres of the cyanamide could provide a novel synthetic route towards nitrogen heterocycles. -

The Reaction of Aminonitriles with Aminothiols: a Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean
life Article The Reaction of Aminonitriles with Aminothiols: A Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean Ibrahim Shalayel , Seydou Coulibaly, Kieu Dung Ly, Anne Milet and Yannick Vallée * Univ. Grenoble Alpes, CNRS, Département de Chimie Moléculaire, Campus, F-38058 Grenoble, France; [email protected] (I.S.); [email protected] (S.C.); [email protected] (K.D.L.); [email protected] (A.M.) * Correspondence: [email protected] Received: 28 September 2018; Accepted: 18 October 2018; Published: 19 October 2018 Abstract: The Strecker reaction of aldehydes with ammonia and hydrogen cyanide first leads to α-aminonitriles, which are then hydrolyzed to α-amino acids. However, before reacting with water, these aminonitriles can be trapped by aminothiols, such as cysteine or homocysteine, to give 5- or 6-membered ring heterocycles, which in turn are hydrolyzed to dipeptides. We propose that this two-step process enabled the formation of thiol-containing dipeptides in the primitive ocean. These small peptides are able to promote the formation of other peptide bonds and of heterocyclic molecules. Theoretical calculations support our experimental results. They predict that α-aminonitriles should be more reactive than other nitriles, and that imidazoles should be formed from transiently formed amidinonitriles. Overall, this set of reactions delineates a possible early stage of the development of organic chemistry, hence of life, on Earth dominated by nitriles and thiol-rich peptides (TRP). Keywords: origin of life; prebiotic chemistry; thiol-rich peptides; cysteine; aminonitriles; imidazoles 1. Introduction In ribosomes, peptide bonds are formed by the reaction of the amine group of an amino acid with an ester function. -

Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
CH. 13 Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution Electrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. Recall: R3 R4 E Y E Y C C + E Y R1 C C R4 R1 C C R4 − R2 R1 δ+ δ R2 R3 R2 R3 In aromatic rings, however, we see substitution of one of the benzene ring hydrogens for an electrophile. H E + E Y + Y H δ+ δ− The mechanism is the same regardless of the electrophile. It involves two steps: (1) Addition of the electrophile to form a high-energy carbocation. (2) Elimination of the proton to restore the aromatic ring system. H H H H slow E Y + Y step 1 + E δ+ δ− high energy arenium ion H H step 2 fast E E + Y + Y H The first step is the slow step since the aromaticity of the benzene ring system is destroyed on formation of the arenium ion intermediate. This is a high energy species but it is stabilized by resonance with the remaining two double bonds. The second step is very fast since it restores the aromatic stabilization. 1 CH. 13 H H H H H H E E E There are five electrophilic aromatic substitution reactions that we will study. (1) Nitration H NO2 H2SO4 + HNO3 (2) Sulfonation H SO3H + H2SO4 (3) Halogenation with bromine or chlorine H X FeX3 X = Br, Cl + X2 (4) Friedel-Crafts Alkylation H R AlX + RX 3 (5) Friedel-Crafts Acylation O H O C AlX3 R + Cl C R 2 CH. -

Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles
Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles Oliver J. Turner,*[a,b] John A. Murphy,*[b] David. J. Hirst[a] and Eric P. A. Talbot*[a]† Abstract: Hydrogen atom transfer-mediated intramolecular C-C coupling reactions between alkenes and nitriles, using PhSiH3 and catalytic Fe(acac)3, are described. This introduces a new strategic bond disconnection for ring-closing reactions, forming ketones via imine intermediates. Of note is the scope of the reaction, including formation of sterically hindered ketones, spirocycles and fused cyclic systems. In the early 1960s, Kwiatek and Seyler first reported the use of metal hydrides as catalysts in the hydrogenation of α,β- unsaturated compounds.[1,2] The discovery by Halpern,[3] later elegantly developed by Norton,[4] that metal-hydride hydrogen atom transfer (HAT) proceeded by a free-radical mechanism opened the door to a wide range of alkene hydrofunctionalisation reactions. But it was the pioneering work by Mukaiyama[5] on the catalytic hydration of alkenes, using Co(acac)2 and oxygen, that sparked wider interest in the field of alkene hydrofunctionalisation. As a result, there now exists an extensive ‘toolkit’ for the addition of hydrogen and a functional group to an alkene with Markovnikov selectivity and high chemo-selectivity using cobalt, manganese and iron complexes.[6,7] Efforts have also been made to extend HAT methodologies to C-C bond formation, both in an intra- and intermolecular fashion: Baran’s group developed a general C-C coupling reaction, utilising electron-deficient alkenes as capable radical acceptors (Scheme 1ai).[8–10] Hydropyridylation of alkenes by intramolecular Minisci reaction was recently demonstrated by Starr,[11] which allows for the formation of structures such as Scheme 1. -

Nitrile Synthesis with Aldoxime Dehydratases: a Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk Chemicals, and Biorefineries
molecules Review Nitrile Synthesis with Aldoxime Dehydratases: A Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk Chemicals, and Biorefineries Pablo Domínguez de María Sustainable Momentum, SL, Av. Ansite 3, 4–6, 35011 Las Palmas de Gran Canaria, Canary Islands, Spain; [email protected]; Tel.: +34-6-0956-5237 Abstract: Nitriles comprise a broad group of chemicals that are currently being industrially produced and used in fine chemicals and pharmaceuticals, as well as in bulk applications, polymer chemistry, solvents, etc. Aldoxime dehydratases catalyze the cyanide-free synthesis of nitriles starting from aldoximes under mild conditions, holding potential to become sustainable alternatives for industrial processes. Different aldoxime dehydratases accept a broad range of aldoximes with impressive high substrate loadings of up to >1 Kg L−1 and can efficiently catalyze the reaction in aqueous media as well as in non-aqueous systems, such as organic solvents and solvent-free (neat substrates). This paper provides an overview of the recent developments in this field with emphasis on strategies that may be of relevance for industry and sustainability. When possible, potential links to biorefineries and to the use of biogenic raw materials are discussed. Keywords: biocatalysis; green chemistry; nitriles; aldoxime dehydratases; sustainability Citation: Domínguez de María, P. Nitrile Synthesis with Aldoxime Dehydratases: A Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk 1. Aldoxime Dehydratases as Biocatalysts for the Cyanide-Free Synthesis of Nitriles Chemicals, and Biorefineries. Nitriles comprise an important group of chemicals that are widely spread in industry Molecules 2021, 26, 4466. in a broad range of sectors, being used as products, solvents, polymers, commodities, or https://doi.org/10.3390/ as starting materials for the production of other chemicals such as amines, amides, etc. -

Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani
Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani To cite this version: Rim Mouselmani. Reduction of Organic Functional Groups Using Hypophosphites. Other. Univer- sité de Lyon; École Doctorale des Sciences et de Technologie (Beyrouth), 2018. English. NNT : 2018LYSE1241. tel-02147583v2 HAL Id: tel-02147583 https://tel.archives-ouvertes.fr/tel-02147583v2 Submitted on 5 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE de DOCTORAT DE L’UNIVERSITE DE LYON EN COTUTELLE AVEC L'UNIVERSITÉ LIBANAISE opérée au sein de l’Université Claude Bernard Lyon 1 École Doctorale de Chimie-École Doctorale des Sciences et Technologies Discipline : Chimie Soutenue publiquement le 07/11/2018, par Rim MOUSELMANI Reduction of Organic Functional Groups Using Hypophosphites Devant le jury composé de Mme. Micheline DRAYE Université Savoie Mont Blanc Rapporteure M. Mohammad ELDAKDOUKI Université Arabe de Beyrouth Rapporteur Mme. Emmanuelle SCHULZ Université Paris 11 examinatrice M. Abderrahmane AMGOUNE Université Lyon 1 Président M. Mahmoud FARAJ Université Internationale Libanaise examinateur Mme. Estelle MÉTAY Université Lyon 1 Directrice de thèse M. Ali HACHEM Université Libanaise Directeur de thèse M. Marc LEMAIRE Université Lyon 1 Membre invité M. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

Ganic Compounds
6-1 SECTION 6 NOMENCLATURE AND STRUCTURE OF ORGANIC COMPOUNDS Many organic compounds have common names which have arisen historically, or have been given to them when the compound has been isolated from a natural product or first synthesised. As there are so many organic compounds chemists have developed rules for naming a compound systematically, so that it structure can be deduced from its name. This section introduces this systematic nomenclature, and the ways the structure of organic compounds can be depicted more simply than by full Lewis structures. The language is based on Latin, Greek and German in addition to English, so a classical education is beneficial for chemists! Greek and Latin prefixes play an important role in nomenclature: Greek Latin ½ hemi semi 1 mono uni 1½ sesqui 2 di bi 3 tri ter 4 tetra quadri 5 penta quinque 6 hexa sexi 7 hepta septi 8 octa octo 9 ennea nona 10 deca deci Organic compounds: Compounds containing the element carbon [e.g. methane, butanol]. (CO, CO2 and carbonates are classified as inorganic.) See page 1-4. Special characteristics of many organic compounds are chains or rings of carbon atoms bonded together, which provides the basis for naming, and the presence of many carbon- hydrogen bonds. The valency of carbon in organic compounds is 4. Hydrocarbons: Compounds containing only the elements C and H. Straight chain hydrocarbons are named according to the number of carbon atoms: CH4, methane; C2H6 or H3C-CH3, ethane; C3H8 or H3C-CH2-CH3, propane; C4H10 or H3C-CH2- CH2-CH3, butane; C5H12 or CH3CH2CH2CH2CH3, pentane; C6H14 or CH3(CH2)4CH3, hexane; C7H16, heptane; C8H18, octane; C9H20, nonane; C10H22, CH3(CH2)8CH3, decane. -

Comparative Responses of Rice (Oryza Sativa) Straw to Urea Supplementation and Urea Treatment
COMPARATIVE RESPONSES OF RICE (ORYZA SATIVA) STRAW TO UREA SUPPLEMENTATION AND UREA TREATMENT M. N. A. Kumar1, K. Sundareshan, E. G. Jagannath S. R. Sampath and P. T. Doyle2 Southern Regional Station, National Dairy Research Institute, Bangalore - 560030, India Summary Twenty five 75% Holstein Friesian cross bred bullocks fed rice straw (Oryza saliva) of long form, were fed with the following five treatments. 1. Rice straw, untreated (RS) 2. RS + water (1:1), stored for 24 hours (WRS) 3. RS (100 kg) + urea solution (4 kg urea/100 litre water) and dried (USRS) 4. RS (100 kg) + urea solution (as in 3) stored in wet condition for 24 hours (UWRS) 5. RS (100 kg) + urea solution (as in 3) stored in pit for 21 days (UTRS). Potential digestibility of treatments of RS was evaluated by monitoring (in vitro) Simulating Rumen like Fermentation (SRLF). The results indicated that Dry Matter Intake (DMI), digestibility of nutrients, N utilization were of the order UTRS > UWRS > USRS > WRS and RS (p < 0.05 to P < 0.01). SRLF index was high (255.84) for UTRS and least (145.58) for USRS. It was interm ediary (199.66) for UWRS. The acetyl content (AC) of UTRS with higher hemicellulose (HCE) dig estibility (80.8%) was low compared to UWRS, USRS, RS and WRS. The acetate content was of the order UTRS < UWRS < USRS < WRS and RS thereby indicating that reduction in acetyl con tent was an index of positive response of urea-treatment of RS. In addition, the ratio of HCE/AC in faeces of UTRS was 0.87 as against the ratios (2.26-2.48) observed in other treatments recording reduction jn AC due to urea-treatinent. -
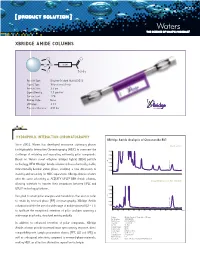
Xbridge Amide Columns
[ PRODUCT SOLUTION ] XBRIDGE AMIDE COLUMNS O O O Si Linker O NH 2 Particle Type: Ethylene Bridged Hybrid [BEH] Ligand Type: Trifunctional Amide Particle Size: 3.5 µm Ligand Density: 7.5 µmol/m2 Carbon Load: 12% Endcap Style: None pH Range: 2-11 Pressure Tolerance: 400 bar HYDROPHILIC INTERacTION CHROMATOGRAPHY XBridge Amide Analysis of Ginsenoside Rb1 Since 2003, Waters has developed innovative stationary phases Orento extract for Hydrophilic Interaction Chromatography [HILIC] to overcome the 0.010 challenge of retaining and separating extremely polar compounds. 0.008 Based on Waters novel ethylene bridged hybrid [BEH] particle 0.006 AU technology, NEW XBridge™ Amide columns utilize a chemically stable, 0.004 0.002 Ginsenoside Rb1 trifunctionally-bonded amide phase, enabling a new dimension in 0.000 stability and versatility for HILIC separations. XBridge Amide columns offer the same selectivity as ACQUITY UPLC® BEH Amide columns, 20 µg/mL Ginsenoside Rb1 standard allowing scientists to transfer their separations between HPLC and 0.010 ® UPLC technology platforms. 0.008 0.006 Designed to retain polar analytes and metabolites that are too polar AU 0.004 to retain by reversed-phase [RP] chromatography, XBridge Amide 0.002 columns facilitate the use of a wide range of mobile phase pH [2 – 11] 0.000 Ginsenoside Rb1 to facilitate the exceptional retention of polar analytes spanning a 0.00. 5 1.01. 5 2.02. 5 3.03. 5 4.04. 5 5.05. 5 6.06. 5 min wide range in polarity, structural moiety and pKa. Column: XBridge Amide, 3.5 µm, 4.6 x 150 mm Part Number: 186004869 Mobile Phase : 80/20 ACN/H2O In addition to enhanced retention of polar compounds, XBridge Flow Rate: 1.4 mL/min Inj. -

Molecular Structure of Wlbb, a Bacterial N-Acetyltransferase Involved in the Biosynthesis †,‡ of 2,3-Diacetamido-2,3-Dideoxy-D-Mannuronic Acid James B
4644 Biochemistry 2010, 49, 4644–4653 DOI: 10.1021/bi1005738 Molecular Structure of WlbB, a Bacterial N-Acetyltransferase Involved in the Biosynthesis †,‡ of 2,3-Diacetamido-2,3-dideoxy-D-mannuronic Acid James B. Thoden and Hazel M. Holden* Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706 Received April 14, 2010; Revised Manuscript Received April 29, 2010 ABSTRACT: The pathogenic bacteria Pseudomonas aeruginosa and Bordetella pertussis contain in their outer membranes the rare sugar 2,3-diacetamido-2,3-dideoxy-D-mannuronic acid. Five enzymes are required for the biosynthesis of this sugar starting from UDP-N-acetylglucosamine. One of these, referred to as WlbB, is an N-acetyltransferase that converts UDP-2-acetamido-3-amino-2,3-dideoxy-D-glucuronic acid (UDP- GlcNAc3NA) to UDP-2,3-diacetamido-2,3-dideoxy-D-glucuronic acid (UDP-GlcNAc3NAcA). Here we report the three-dimensional structure of WlbB from Bordetella petrii. For this analysis, two ternary structures were determined to 1.43 A˚resolution: one in which the protein was complexed with acetyl-CoA and UDP and the second in which the protein contained bound CoA and UDP-GlcNAc3NA. WlbB adopts a trimeric quaternary structure and belongs to the LβH superfamily of N-acyltransferases. Each subunit contains 27 β-strands, 23 of which form the canonical left-handed β-helix. There are only two hydrogen bonds that occur between the protein and the GlcNAc3NA moiety, one between Oδ1 of Asn 84 and the sugar C-30 amino group and the second between the backbone amide group of Arg 94 and the sugar C-50 carboxylate.