7 CFR Ch. I (1–1–13 Edition)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
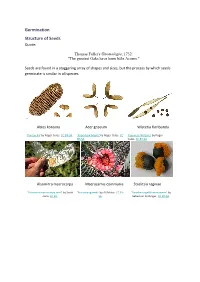
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil. -

Agricultural Marketing Service, USDA § 201.56–10
Agricultural Marketing Service, USDA § 201.56–10 (A) One or more essential structures and become thin, leaf-like, and photo- impaired as a result of decay from pri- synthetic. mary infection. (3) Shoot system: The hypocotyl (B) Albino. elongates carrying the cotyledons above the soil surface. The epicotyl [59 FR 64504, Dec. 14, 1994] usually does not show any development § 201.56–8 Flax family, Linaceae. within the test period. Areas of yel- lowish pigmentation may develop on Kind of seed: Flax. the hypocotyl in cotton. (a) General description. (4) Root system: A primary root, with (1) Germination habit: Epigeal dicot. secondary roots usually developing (Due to the mucilaginous nature of the within the test period. Areas of yel- seed coat, seedlings germinated on lowish pigmentation may develop on blotters may adhere to the blotter and the root in cotton. appear to be negatively geotropic.) (b) Abnormal seedling description. (2) Food reserves: Cotyledons which (1) Cotyledons: expand and become photosynthetic. (i) Less than half of the original cot- (3) Shoot system: The hypocotyl yledon tissue remaining attached. elongates carrying the cotyledons (ii) Less than half of the original cot- above the soil surface. The epicotyl yledon tissue free of necrosis or decay. usually does not show any development (Remove any attached seed coats at within the test period. the end of the test period for evalua- (4) Root system: A primary root, with tion of cotyledons.) secondary roots usually developing (2) Epicotyl: within the test period. (i) Missing. (May be assumed to be (b) Abnormal seedling description. present if both cotyledons are intact.) (1) Cotyledons: (ii) [Reserved] (i) Less than half of the original cot- (3) Hypocotyl: yledon tissue remaining attached. -

Federal Aviation Agency
FEDERAL REGISTER VOLUME 30 • NUMBER 117 Friday, June 18,1965 • Washington, D.C. Pages 7863-7938 Agencies in this issue— Agricultural Research Service Area Redevelopment Administration Atomic Energy Commission Civil Aeronautics Board Civil Service Commission Consumer and Marketing Service Federal Aviation Agency Federal Communications Commission Federal Maritime Commission Federal Trade Commission Food and Drug Administration Interstate Commerce Commission Land Management Bureau Securities and Exchange Commission Detailed list of Contents appears inside. Subscriptions Now Being Accepted S L I P L A W S 89th Congress, 1st Session 1965 Separate prints of Public Laws, published immediately after enactment, with marginal annotations and legislative history references. Subscription Price: $12.00 per Session Published by Office of the Federal Register, National Archives and Records Service, General Services Administration Order from Superintendent of Documents, U.S. Government Printing Office, Washington, D.C.,' 20402 Published dally, Tuesday through Saturday (no publication on Sundays, Monday», or FEDERAL®REGISTER on the day after an official Federal holiday), by the Office of the Federal Register, National Area Code 202 Phone 963-3261 A1®1117®8 a n d R ecords Service, G eneral Services A d m in istra tio n (m ail address Nations _ . , _ _ Archives Building, Washington, D.C. 20408), p u r s u a n t to the authority contained in tne Federal Register Act, approved July 26, 1935 (49 Stat. 500, as amended; 44 U.S.C., ch. 8B), under regulations prescribed by the Admin istrative Committee of the Federal Register, approved by the President (1 CFR Ch. I). Distribution is made only by the Superintendent or Documents, Government Printing Office, Washington, D.C. -

Journal of the American Horticultural Society, Inc
Journal of the American Horticultural Society, Inc. 901 NORTH WASHINGTON STREET, ALEXANDRIA, VIRGINIA 22314 For United Horticulture ... The particular objec.ts and business of the American Horticultural Society a.ye to promote and encourage national in·terest in scientific research and education in horticulture in all of its branches. 1970-71 EXECUTIVE COMMITTEE (*) President Treasurer DR. DAVID G. LEACH (1971) MR. JOHN M. PATEK (1972) 1674 Trinity Road President North Madison, Ohio 44057 Color Data, Inc. 434 Mount Airy Drive First Vice President Rochester, New York 14617 MRS. ERNESTA D. BALLARD (1973) Director, Pennsylvania MembeT of the Board Horticultural Society DR. HENRY M. CATHEY (1973) 325 Walnut Street Leader, Ornamental Investigations Philadelphia, Pennsylvania 19106 Agricultural Research Service Second V ice President U.S. Department of Agriculture Beltsville, \!faryland 20705 MR. FREDERICK J. CLOSE (1971) 6189 Shore Drive Immediate Past President North Madison, Ohio 44057 MR. FRED C. GALLE (1971) (U) Secretary Director of Horticulture DR. GEORGE H. M. LAWRENCE (1971) Callaway Gardens 390 Forge Road Pine Mountain, Georgia 31822 East Greenwich, Rhode Island 02818 MR. O. KEISTER EVANS Executive Director Washington, D. C. (e) Members of the 1970-71 Board of Directors per bylaw provision. ( ..) Ex officio, and without vote. THE AMERICAN HORTICULTURAL MAGAZINE is the official publication of The American Horticultural Society and is issued during the Winter, Spring, Summer, and Fall quarters. The magazine is included as a benefit of membership in The American Horticultural Society, individual membership dues being $15 .00 a year. THE AMERICAN HORTICULTURAL MAGAZINE is devoted to the dissemination of knowledge in the science and art of growing ornamental plants, fruits, vegetables, and related subjects. -
Dicots Versus Monocots
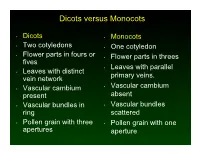
Dicots versus Monocots • Dicots • Monocots • Two cotyledons • One cotyledon • Flower parts in fours or • Flower parts in threes fives • Leaves with parallel • Leaves with distinct primary veins. vein network • Vascular cambium • Vascular cambium present absent • Vascular bundles in • Vascular bundles ring scattered • Pollen grain with three • Pollen grain with one apertures aperture Fruit and Seed Dispersal • Water Dispersal ! Some fruits contain trapped air. ! Some have a waxy coating. ! Both means help the seed to float. ! Example- coconut • Mechanical Ejection of Seeds ! Capsules dry and split in a way that flings the seeds far from the parent plant. ! Example witch hazel Fruit and Seed Dispersal • Wind Dispersal ! Small and Lightweight seeds. ! May have attachments like wings or hairs to help give them lift. ! Example- maple, ash, dandelion • Animal Dispersal ! Seeds can pass through an animal’s digestive tract. ! Some fruits and seeds have spines or thorns that catch in fur or feathers. ! Oils attract ants. Corn- pollinated by wind Birds, Butterflies, and Bats How Seeds Form • Pollination is the transfer of pollen from an anther to a stigma. • This may occur by wind or pollinating insects, birds or other animals. • Wind pollinated flowers usually lack showy floral parts and nectar since they don’t need to attract a pollinator. • Their non-showy flowers, however, do produce a lot of pollen to increase the likelihood that a pollen grain will land on a receptive stigma. How Seeds Form • Brightly colored flowers, those with distinct markings or patterns on petals, containing fragrance or nectar, are most likely pollinated by insects, birds, or other animals. -

Seedling Emergence Patterns
1 Seedling emergence patterns Learning objective: To be able to recognize the two major seedling emergence patterns. Background information: There are two common patterns of seedling emergence. The most common pattern is where the cotyledons are raised above ground as the seedling emerges. The seedling forms a hypocotyl hook that pushes through the soil. Once the seedling reaches the light, the hook opens to create a straight seedling. This pattern is called epigeous. In the second pattern, the cotyledons remain underground and the stem (epicotyl) emerges above ground. This pattern is called hypogeous. Large seeded plants like Kentucky coffeetree and oaks tend to have hypogeous germination. The cotyledons function as the repository for food reserves in the seed. During seed development the plant stores food in the cotyledons that the seedling will use for emergence. Once the seedling emerges into the sunlight, it can begin to make its own food through photosynthesis. 2 In the group of plants covered in this website, the oaks, nuts (Ohio buckeye, black walnut and pecan), paw paw, and Kentucky coffeetree have a hypogeous seedling emergence pattern. For black walnut, pecan, and paw paw, the cotyledons never emerge from the seed. All the other plant species on the website have a epigeous seedling emergence pattern. An easy comparison can be made using Kentucky coffeetree and honeylocust. They are both in the legume family and only have physical dormancy. The seeds are easy to collect, are large and store for a very long time so the experiment can be conducted any time of the year. Procedures: 1. -

Germination Studies of Some Trees and Shrubs, 1928
Proceedings of the Iowa Academy of Science Volume 35 Annual Issue Article 30 1928 Germination Studies of Some Trees and Shrubs, 1928 L. H. Pammel C. M. King Let us know how access to this document benefits ouy Copyright ©1928 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Pammel, L. H. and King, C. M. (1928) "Germination Studies of Some Trees and Shrubs, 1928," Proceedings of the Iowa Academy of Science, 35(1), 184-197. Available at: https://scholarworks.uni.edu/pias/vol35/iss1/30 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Pammel and King: Germination Studies of Some Trees and Shrubs, 1928 GERMINATION STUDIES OF SOME TREES AND SHRUBS, 1928 L. H. p AM MEL AND C. M. KING The studies for the spring of 1928 are a continuation of previous work and constitute the eleventh contribution to the series. In the present paper seedlings of twenty-three species of plants are de scribed, the germination periods noted, and the seedlings figured. These plants include both native and introduced forms. The seeds were contributed by Arnold Arboretum and other sources, many being the collections of the senior author in the field. Pinaceae Pinus echinata Mill. Yellow Pine Pinus heterophylla (Ell.) Sudworth, Slash Pine, Cuban Pine Juniperus phoenicia L. -

Flower Speciesspecies
13 June 2011 ISTA GERMINATION SEMINAR GERMINATIONGERMINATION CHARACTERISTICSCHARACTERISTICS OFOF FLOWERFLOWER SPECIESSPECIES RITA ZECCHINELLI - ISTA FLOWER SEED TESTING COMMITTEE Luca Flower species in the ISTA Rules: . 352 species . 192 genera . 55 families 13 June 2011 ISTA GERMINATION SEMINAR RITA ZECCHINELLI DICOTYLEDON FAMILIES Family N°genera N°species Family N°genera N°species Acanthaceae 1 1 Hypericaceae 1 1 Aizoaceae 1 1 Iridaceae 1 1 Amaranthaceae 3 6 Lamiaceae 14 21 Apiaceae 3 4 Linaceae 1 4 Apocynaceae 1 1 Malvaceae 6 7 Araliaceae 2 2 Nyctaginaceae 1 1 Asclepiadeaceae 1 1 Onograceae 2 4 Asteraceae 49 86 Papaveraceae 3 7 MONOCOTYLEDON FAMILIES Balsaminaceae 1 2 Plumbaginaceae 4 8 Family N°genera N°species Begoniaceae 1 2 Polemoniaceae 3 5 Amaryllidaceae 1 1 Boraginaceae 6 10 Polygonaceae 1 1 Asparagaceae 1 2 Brassicaceae 11 22 Portulaceae 1 1 Asphodelaceae 1 1 Campanulaceae 3 13 Primulaceae 3 12 Liliaceae 1 1 Capparidaceae 1 1 Proteaceae 1 1 Poaceae 2 2 Caryophyllaceae 6 16 Ranuncolaceae 7 20 Chenopodiaceae 1 1 Resedaceae 1 1 Cistaceae 1 1 Rosaceae 2 3 Convolvulaceae 2 5 Rutaceae 1 1 Crassulaceae 1 3 Saxifragaceae 1 1 Dipsacaceae 1 2 Scrophulariaceae 10 22 Fabaceae 4 8 Solanaceae 9 15 Gentianaceae 1 1 Tropaeolaceae 1 3 Geraniaceae 2 2 Valerianceae 1 1 Gesneriaceae 2 2 Verbenaceae 2 4 Hydrophyllaceae 3 4 Violaceae 1 3 13 June 2011 ISTA GERMINATION SEMINAR RITA ZECCHINELLI What flower species have in common? . ornamental use . fast changing trends in the market . for seed testing laboratories - less experience in standardized -

Plant Disease
report on RPD No. 911 PLANT April 2002 DEPARTMENT OF CROP SCIENCES DISEASE UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN ROOT ROTS OF PEA Root rots occur wherever peas are grown in the world. Crop losses are probably greater from root diseases than from any other type of pea disease. Damage is usually most severe in wet seasons. Low-lying areas and slowly drained fields or gardens suffer greater losses than well-drained, fertile ones. Root rot generally occurs in patches that progressively enlarge during the season. Root rot may start when the pea plant is in the pre- or post- emergent seedling stage. Death soon follows such early infections, resulting in a poor stand. Root decay generally begins on the fine feeder roots and progresses gradually to the main taproot. Sometimes, however, the taproot is the first to be attacked. In some cases all roots are destroyed, leaving only remnants below the attachment of the seed. Root-rotting fungi can also reduce the quality of shelled peas. Infected plants produce peas which are irregular in size and variable in maturity with a lowered sugar content. Root rot of pea may be caused by any one or a combination of several common soil fungi. The most common pathogens are Aphanomyces euteiches, Pythium ultimum, Fusarium Figure 1. Healthy pea plant on left and plants solani f. sp. pisi, and Rhizoctonia solani. Other fungi that infected with Aphanomyces root rot on right. can be associated with pea root rots include Thielaviopsis basicola, Fusarium oxysporum, Ascochyta pinodella, and Sclerotinia sclerotiorum. Aphanomyces euteiches and Pythium ultimum are probably the most important of the fungal species reported. -

7 CFR Ch. I (1–1–12 Edition) § 201.56–6
§ 201.56–6 7 CFR Ch. I (1–1–12 Edition) (C) No leaf. (B) Short, thick, and grainy. (D) Leaf extending less than halfway (C) No leaf. up into the coleoptile. (D) Leaf extending less than halfway (E) Leaf extensively shredded or up into the coleoptile. split. (E) Leaf extensively shredded or (F) Spindly or watery. split. (G) Deep open cracks in the (F) Spindly or watery. mesocotyl. (G) Deep open cracks in the (ii) Root: mesocotyl. (A) None. (ii) Root: (B) Damaged or weak primary root (A) Missing or defective primary root with less than two strong secondary even if other roots are present. roots. (B) Spindly, stubby, or watery pri- (iii) Seedling: mary root. (A) Decayed at point of attachment (iii) Seedling: to the scutellum. (A) Decayed at point of attachment (B) One or more essential structures to the scutellum. impaired as a result of decay from pri- (B) One or more essential structures mary infection. impaired as a result of decay from pri- (C) Albino. mary infection. (e) Grasses and millets. (C) Albino. (1) General description. (D) Yellow (when grown in light). (i) Germination habit: Hypogeal (E) Endosperm obviously detached monocot. from the root-shoot axis (e.g. kernel (ii) Food reserves: Endosperm. The lifted away by the growing shoot). scutellum is a modified cotyledon which is in direct contact with the [59 FR 64501, Dec. 14, 1994, as amended at 65 endosperm. During germination the FR 1708, Jan. 11, 2000] scutellum remains inside the seed to absorb nutrients from the endosperm § 201.56–6 Legume or pea family, Fabaceae (Leguminosae).