Aphanomyces Root Rot in Pulse Crops Entire Root System Is Colonized
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
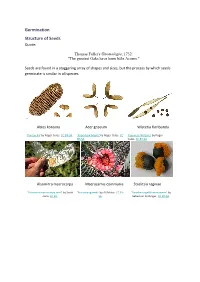
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -
Published Version
PUBLISHED VERSION Rays H. Y. Jiang ... Vincent Bulone ... et al. Distinctive expansion of potential virulence genes in the genome of the oomycete fish pathogen Saprolegnia parasitica PLoS Genetics, 2013; 9(6):e1003272-1-e1003272-20 © 2013 Jiang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Originally published at: http://doi.org/10.1371/journal.pgen.1003272 PERMISSIONS http://creativecommons.org/licenses/by/4.0/ http://hdl.handle.net/2440/97179 Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen Saprolegnia parasitica Rays H. Y. Jiang1., Irene de Bruijn2¤a., Brian J. Haas1., Rodrigo Belmonte2,3,LarsLo¨ bach2, James Christie2,3, Guido van den Ackerveken4, Arnaud Bottin5, Vincent Bulone6, Sara M. Dı´az-Moreno6, Bernard Dumas5, Lin Fan1, Elodie Gaulin5, Francine Govers7,8, Laura J. Grenville-Briggs2,6, Neil R. Horner2, Joshua Z. Levin1, Marco Mammella9, Harold J. G. Meijer7, Paul Morris10, Chad Nusbaum1, Stan Oome4, Andrew J. Phillips2, David van Rooyen2, Elzbieta Rzeszutek6, Marcia Saraiva2, Chris J. Secombes3, Michael F. Seidl8,11, Berend Snel8,11, Joost H. M. Stassen4, Sean Sykes1, Sucheta Tripathy12, Herbert van den Berg2, Julio C. Vega-Arreguin13, Stephan Wawra2, Sarah K. Young1, Qiandong Zeng1, Javier Dieguez- Uribeondo14, Carsten Russ1", Brett M. Tyler12¤b", Pieter van West2*" 1 Broad Institute -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

Host-Pathogen Interactions in Root Infecting Oomycete Species
Host-Pathogen Interactions in Root Infecting Oomycete Species Sara Hadji Mollahossein Faculty of Natural Resources and Agricultural Sciences Department of Forest Mycology and Plant Pathology Uppsala Doctoral Thesis Swedish University of Agricultural Sciences Uppsala 2015 Acta Universitatis Agriculturae Sueciae 2015:9 Cover: Pea plant infected with Phytophthora pisi (left), control plant (right). Sporangia releasing zoospores (left) and oospores (right) in infected root tissue. (Photo: Sara Hosseini) ISSN 1652-6880 ISBN (print version) 978-91-576-8216-1 ISBN (electronic version) 978-91-576-8217-8 © 2015 Sara Hadji Mollahossein, Uppsala Print: SLU Service/Repro, Uppsala 2015 Host-Pathogen Interactions in Root Infecting Oomycete Species Abstract The oomycetes include some of the most devastating pathogens on both cultivated crops and wild plants. In the genus Phytophthora some closely related species have a broad host range, while others are very host specific. The aim of this project was to gain an understanding of the mechanisms underlying the differentiation of a subgroup of root-infecting Phytophthora species and to gain knowledge about the plant immune responses triggered by distantly related oomycetes that adapted to the same legume host. We investigated the zoospore chemotaxis of legume-root infecting Phytophthora species to different isoflavonoid compounds and explored a possible connection to host preference. Our results showed that specific chemotaxis towards host isoflavones is of limited importance in Phytophthora sojae and Phytophthora vignae, while, specific chemotaxis of Phytophthora pisi and Phytophthora niederhauserii indicated an adaptation to their pathogenicity on the host and lack of pathogenicity on non-host plants. The comparative proteomic study of P. pisi and P. -

Genetic Mapping of Resistance to Aphanomyces Root Rot in Alfalfa *E
Genetic mapping of resistance to Aphanomyces root rot in alfalfa15 Samac, D.A.*, Dornbusch, M. R., Bucciarelli, B., Miller, S. S. and Yu, L.-X. USDA-Agricultural Research Service *e-mail: [email protected] KEYWORDS: Aphanomyces euteiches, genotyping by sequencing, NBS-LRR resistance genes Aphanomyces root rot (ARR), caused by the oomycete Aphanomyces euteiches, is one of the most important yield-limiting factors in production of legumes. In Europe, it is the main limiting factor for pea production while in the United States it is one of the most important diseases of alfalfa and pea (Gaulin et al., 2007). The disease causes stunting and death of alfalfa seedlings with complete stand loss occurring in wet and poorly drained soils. Non-lethal damage to seedling roots reduces forage yields and winter survival. The pathogen can infect adult plants during wet periods to cause loss of feeder roots and root nodules, reducing nitrogen fixation, forage yield, and stand life. Varieties with resistance to ARR became widely available in the 1990s; however, failure of resistant varieties identified a second race of the pathogen. Recent reports of failure of varieties with resistance to both race 1 and race 2 suggest that additional pathogenic races are present in alfalfa production fields. The goals of this research were to: (i) determine the prevalence of race 1 and race 2 strains in Minnesota and New York; (ii) identify novel strains of the pathogen; (iii) characterize the race-specific resistance to Aphanomyces root rot; and (iv) map resistance genes in order to facilitate breeding for resistance and to clarify race/resistance gene relationships. -

Development of New Genome-Informed Genotyping Tools for Aphanomyces Astaci
Development of new genome-informed genotyping tools for Aphanomyces astaci Submitted by Diana Minardi to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Biological Sciences In May 2017 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. Signature: ………………………………………………………….. 1 Acknowledgements I would like to sincerely thank my supervisors Dr Mark van der Giezen, Dr David Studholme, and Dr Birgit Oidtmann for their invaluable guidance and support during these 3 years (plus a bit) of PhD, both practically and in the writing of the thesis. I would like to thank Cefas and the University of Exeter for funding the project and for giving me the opportunity to embrace this challenge and adventure. I would like to thank all of the University of Exeter “Biocat” lab and office, with whom I’ve spent most of my coffee time. But especially Mirella (for the laughs and cries) and Simone (for the sweaty runs around campus). I would also like to thank Chiara for her constant support, encouragement, and beer. Finally, I would like to thank Tony, for his patience, and my family, for their “overseas” support: I’ve finally finished “the shrimp” book! 2 Abstract Aphanomyces spp. are water moulds, eukaryotic fungus-like organisms, belonging to the class Oomycota. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil. -

Agricultural Marketing Service, USDA § 201.56–10
Agricultural Marketing Service, USDA § 201.56–10 (A) One or more essential structures and become thin, leaf-like, and photo- impaired as a result of decay from pri- synthetic. mary infection. (3) Shoot system: The hypocotyl (B) Albino. elongates carrying the cotyledons above the soil surface. The epicotyl [59 FR 64504, Dec. 14, 1994] usually does not show any development § 201.56–8 Flax family, Linaceae. within the test period. Areas of yel- lowish pigmentation may develop on Kind of seed: Flax. the hypocotyl in cotton. (a) General description. (4) Root system: A primary root, with (1) Germination habit: Epigeal dicot. secondary roots usually developing (Due to the mucilaginous nature of the within the test period. Areas of yel- seed coat, seedlings germinated on lowish pigmentation may develop on blotters may adhere to the blotter and the root in cotton. appear to be negatively geotropic.) (b) Abnormal seedling description. (2) Food reserves: Cotyledons which (1) Cotyledons: expand and become photosynthetic. (i) Less than half of the original cot- (3) Shoot system: The hypocotyl yledon tissue remaining attached. elongates carrying the cotyledons (ii) Less than half of the original cot- above the soil surface. The epicotyl yledon tissue free of necrosis or decay. usually does not show any development (Remove any attached seed coats at within the test period. the end of the test period for evalua- (4) Root system: A primary root, with tion of cotyledons.) secondary roots usually developing (2) Epicotyl: within the test period. (i) Missing. (May be assumed to be (b) Abnormal seedling description. present if both cotyledons are intact.) (1) Cotyledons: (ii) [Reserved] (i) Less than half of the original cot- (3) Hypocotyl: yledon tissue remaining attached. -

Federal Aviation Agency
FEDERAL REGISTER VOLUME 30 • NUMBER 117 Friday, June 18,1965 • Washington, D.C. Pages 7863-7938 Agencies in this issue— Agricultural Research Service Area Redevelopment Administration Atomic Energy Commission Civil Aeronautics Board Civil Service Commission Consumer and Marketing Service Federal Aviation Agency Federal Communications Commission Federal Maritime Commission Federal Trade Commission Food and Drug Administration Interstate Commerce Commission Land Management Bureau Securities and Exchange Commission Detailed list of Contents appears inside. Subscriptions Now Being Accepted S L I P L A W S 89th Congress, 1st Session 1965 Separate prints of Public Laws, published immediately after enactment, with marginal annotations and legislative history references. Subscription Price: $12.00 per Session Published by Office of the Federal Register, National Archives and Records Service, General Services Administration Order from Superintendent of Documents, U.S. Government Printing Office, Washington, D.C.,' 20402 Published dally, Tuesday through Saturday (no publication on Sundays, Monday», or FEDERAL®REGISTER on the day after an official Federal holiday), by the Office of the Federal Register, National Area Code 202 Phone 963-3261 A1®1117®8 a n d R ecords Service, G eneral Services A d m in istra tio n (m ail address Nations _ . , _ _ Archives Building, Washington, D.C. 20408), p u r s u a n t to the authority contained in tne Federal Register Act, approved July 26, 1935 (49 Stat. 500, as amended; 44 U.S.C., ch. 8B), under regulations prescribed by the Admin istrative Committee of the Federal Register, approved by the President (1 CFR Ch. I). Distribution is made only by the Superintendent or Documents, Government Printing Office, Washington, D.C. -

Root Rots in Pulses
CROP DEVELOPMENT CENTRE Root Rots in Pulses Michelle Hubbard, Agriculture and Agri-Food Canada, Swift Current Research Scientist, Pulse Pathology Co-authors: Sabine Banniza, Zakir Hossain, Sherrilyn Phelps, Syama Chatterton, Luke Bainard & Barb Ziesman What is root rot? Caused by micro-organisms Range of pathogens • Fusarium species • Pythium species • Rhizoctonia solani • Aphanomyces euteiches Pea and lentil do not like wet feet High soil moisture can cause • ↓ root and shoot growth • yellowing • ↓ nodulation Stressed plants are more susceptible to infection Normal soil Water- moisture saturated Peas in sterile field soil Root rot is a complex Fusarium species Pythium species Rhizoctonia solani Aphanomyces euteiches And it is complicated! Wider host range True fungi • Fusarium spp. (e.g. solani, • Fusarium spp. avenaceum, acuminatum, graminearum) • Rhizoctonia solani • Rhizoctonia solani • Pythium spp. Fungus-like organisms (oomycetes) Attack only specific plants: • Pythium spp. • Fusarium oxysporum f.sp. pisi, f.sp. ciceris or f. sp. • Aphanomyces lentis, F. virgulifome euteiches • Aphanomyces euteiches • Phytophthora spp. • Phytophthora spp Fusarium Courtesy of S. Chatterton, AAFC Infects many different plants Courtesy of F. Dokken-Bouchard, SMA Aphanomyces Oospores Infects pea and lentil Oospores = resting spores • More vulnerable after they germinate Zoospores: can swim short distances Courtesy of S. Chatterton, AAFC Courtesy of F. Dokken-Bouchard, SMA Aphanomyces Oospores EveryInfects time pea aand plant lentil gets infected,Oospores -
Meeting Program
Oomycete Molecular Genetics Network Meeting March 10 to March 12 2013 Asilomar Conference Grounds Pacific Grove, CA Oomycete Molecular Genetics Network Meeting March 10 to March 12, 2013 Asilomar Conference Grounds Pacific Grove, CA The Oomycete Molecular Genetics Research Network was initially funded by an NSF Research Coordination Network grant in 2001. The purpose of our annual meeting is to promote communication and collaboration, and minimize duplication of effort within the worldwide Oomycete molecular genetics community. The oomycete molecular genetics community now numbers well over one hundred labs from across the world, and papers on oomycetes attract a readership that goes well beyond the community itself. The Oomycete Molecular Genetics Conference alternates between USA and Eurasia, and returns this year to Asilomar, California. This year’s meeting will cover some of the latest research on Effector Biology, Genomics and Oomycete evolution and population biology. With over 100 attendees expected, this meeting represents the largest network meeting held in the US. Committee Chairs John McDowell Mark Gijzen Associate Professor Research Scientist Plant Pathology, Physiology and Weed Science Agriculture and Agri-food Canada Virginia Tech, Blacksburg, VA 24061 London ON, Canada NSV4T3 Meeting Logistics Facilities Coordinator Joel Shuman Paul Morris Project Manager Professor, Biological Sciences Plant Pathology, Physiology and Weed Science Bowling Green State University Virginia Tech, Blacksburg, VA24061 Bowling Green, OH 43403 Cover: Confocal image of GFP-expressing Phytophthora sojae at 12h after inoculation of soybean hypocotyls. Image courtesy of Kai-Tao from the lab of Yuanchao Wang, Nanjing Agricultural University, Nanjing, China. Meeting Sponsors This meeting is supported in part by NSF grant MCB-0639226 to Brett Tyler OMGN 2013 Program Location: Talks will be held at Kiln on Sunday Morning and at Fred Farr for all other sessions. -

APHANOMYCES ROOT ROT in PEAS and LENTILS in Western Canada
APHANOMYCES ROOT ROT IN PEAS AND LENTILS in Western Canada albertapulse.com manitobapulse.ca saskpulse.com @albertapulse @mbpulsegrowers @saskpulse ROOT ROTS Root rot in peas and lentils is caused by a complex of diseases that affect the belowground portion of the developing plant, leading to poor performing pulse crops. The organisms that cause the disease are seed- or soil-borne and can infect the plant at any stage. Unfortunately, once root rot has set in, there is nothing that can be done. Understanding the disease, identifying the risks for root rot infection, and thorough planning for prevention are the only current options. Aphanomyces Root Rot DNA testing conducted on root rots in Alberta, Manitoba Aphanomyces root rot is caused by Aphanomyces and Saskatchewan in 2017 (see survey results below), euteiches, a highly specialized pathogen of legumes. show that Aphanomyces is a common cause of root rots While this pathogen has a number of legume host plants, in pulse crops in the Prairies. Aphanomyces can infect peas and lentils are the most susceptible pulse crops to at any time in the growing season and spores persist for infection. Faba beans and sainfoin exhibit good partial PDQ\\HDUVLQWKHVRLOPDNLQJLWWKHPRVWGLI¿FXOWFDXVH (quantitative) resistance to Aphanomyces, and chickpeas of root rot to manage (and therefore the most serious are considered moderately resistant. Soybeans and among the root rot pathogens). While research is being fenugreek are both non-host crops to A. euteiches. undertaken in Alberta, Manitoba and Saskatchewan, there is currently no reliable prevention or cure. Susceptibility of dry beans and alfalfa to Aphanomyces root rot infection varies among the different varieties.