Seedlings of Dicotyledons.Structure, Development, Types
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
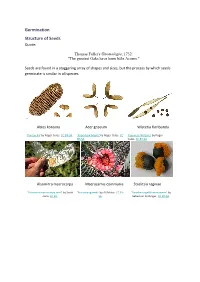
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -

Asian Pacific Journal of Tropical Disease
Asian Pac J Trop Dis 2016; 6(6): 492-501 492 Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Disease journal homepage: www.elsevier.com/locate/apjtd Review article doi: 10.1016/S2222-1808(16)61075-7 ©2016 by the Asian Pacific Journal of Tropical Disease. All rights reserved. Phytochemistry, biological activities and economical uses of the genus Sterculia and the related genera: A reveiw Moshera Mohamed El-Sherei1, Alia Yassin Ragheb2*, Mona El Said Kassem2, Mona Mohamed Marzouk2*, Salwa Ali Mosharrafa2, Nabiel Abdel Megied Saleh2 1Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Giza, Egypt 2Department of Phytochemistry and Plant Systematics, National Research Centre, 33 El Bohouth St., Dokki, Giza, Egypt ARTICLE INFO ABSTRACT Article history: The genus Sterculia is represented by 200 species which are widespread mainly in tropical and Received 22 Mar 2016 subtropical regions. Some of the Sterculia species are classified under different genera based Received in revised form 5 Apr 2016 on special morphological features. These are Pterygota Schott & Endl., Firmiana Marsili, Accepted 20 May 2016 Brachychiton Schott & Endl., Hildegardia Schott & Endl., Pterocymbium R.Br. and Scaphium Available online 21 Jun 2016 Schott & Endl. The genus Sterculia and the related genera contain mainly flavonoids, whereas terpenoids, phenolic acids, phenylpropanoids, alkaloids, and other types of compounds including sugars, fatty acids, lignans and lignins are of less distribution. The biological activities such as antioxidant, anti-inflammatory, antimicrobial and cytotoxic activities have Keywords: been reported for several species of the genus. On the other hand, there is confusion on the Sterculia Pterygota systematic position and classification of the genus Sterculia. -

Pharmacognosy, Phytochemical Study and Antioxidant Activity of Sterculia Rubiginosa Zoll
Pharmacogn J. 2018; 10(3):571-575. A Multifaceted Journal in the field of Natural Products and Pharmacognosy Original Article www.phcogj.com | www.journalonweb.com/pj | www.phcog.net Pharmacognosy, Phytochemical Study and Antioxidant Activity of Sterculia rubiginosa Zoll. Ex Miq. Leaves Rini Prastiwi1,2*, Berna Elya2, Rani Sauriasari3, Muhammad Hanafi4, Ema Dewanti1 ABSTRACT Introduction: Sterculia rubiginosa Zoll ex.Miq leaves have been used as traditional medicine in Indonesia. There is no report about pharmacognosy and phytochemical study with this plant.Objective: The main aim of this research is to establish pharmacognosy, phytochemical study and antioxidant activity of Sterculia rubiginosa Zoll.ex. Miq. Leaves. The plant used to cure many diseases of Indonesia. Methods: In the present study, pharmacognosy and phyto- 1,2* chemical study of plant material were performed as per the Indonesian Herb Pharmacopoeia. Rini Prastiwi , Berna Results: Microscopy powder of Sterculia rubiginosa Zoll.ex. Miq. Leaves shows star shape Elya2, Rani Sauriasari3, trichoma as a specific fragment. Physicochemical parameters including total ash (17.152 %), Muhammad Hanafi4, acid-insoluble ash (0.922 %), water-soluble extractive (1.610 % w/w), alcohol-soluble extractive Ema Dewanti1 (4.524 % w/w), hexane-soluble extractive (4.005 % w/w), and ethyl acetate-soluble extractive (3.160 % w/w) were evaluated. Phytochemical screening of ethanol extracts showed the 1Department of Pharmacognosy- presence of tannins, flavonoids, alkaloids, steroids-terpenoids, glycosides, and phenols. And Phytochemistry, Faculty of Pharmacy absent of saponins and Anthraquinones. Antioxidant activity with IC50 157, 4665 ppm and and Science Muhammadiyah Prof.Dr. flavonoid total was 59.436 mg/g quercetin equivalent. -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

Foliar Architecture of Indian Members of the Family Sterculiaceae and Its Systematic Relevance”
PROJECT REPORT: FINAL UGC–MRP: F. No. 40–327/2011 (SR); dt. 30th June, 2011 TITLE OF THE PROJECT “FOLIAR ARCHITECTURE OF INDIAN MEMBERS OF THE FAMILY STERCULIACEAE AND ITS SYSTEMATIC RELEVANCE” PRINCIPAL INVESTIGATOR DR. DEBABRATA MAITY Assistant Professor Department of Botany Taxonomy & Biosystematics Laboratory University of Calcutta 35, Ballygunge Circular Road, Kolkata – 700 019 INDEX TO FIGURES FIGURE DETAILS FOLLOWED NO. PAGE NO. Fig.1 Presentation and illustration of Heritiera fomes 9 Fig.2 Presentation and illustration of Heritiera fomes continued 9 Fig.3 Transverse section of internode and node 10 Fig.4 Transverse section of petiole at different topography 10 Fig.5 Leaf shapes and major venation patterns in different members of 10 Sterculiaceae Fig.6 Types of Minor venation in different members of Sterculiaceae 11 Fig.7 Types of Margin and marginal venation in different members of 11 Sterculiaceae Fig.8 Types of vein ends in different members of Sterculiaceae 12 Fig.9 Types of trichomes, crystals and stomata in different members of 12 Sterculiaceae CONTENTS Titles Page No. 1. Introduction……………………………………………………………………..... 1 2. Review of literatures…………………………………………………………...... 3 3. Objectives..…………………………………………………………..................... 5 4. Materials and Methods…………………………………………………………... 6 4.1 Materials 4.2 Methods 4.2.1 Morphology 4.2.2 Anatomy 4.2.3 Venation of lamina 4.2.4 Dermal features and inclusions 4.2.5 Morphometric analysis 4.2.6 Key to the species 8. Observations……………………………………………………………………… 9 I. Study of stem and petiole 8.1 Internodal anatomy 8.2 Nodal anatomy 8.3 Petiolar anatomy II. Study of lamina 8.4 Laminar shape 8.5 Laminar venation 8.5.1 Major venation 8.5.2 Minor venation 8.5.3 Margin and Marginal venation 8.5.4 Free vein endings 8.6 Dermal features and inclusions 8.6.1 Trichomes 8.6.2 Scales 8.6.3 Stomata 8.6.4 Crystals III. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil. -

Phytochemical Screening of Active Secondary Metabolites Present in the Leaves Extracts of Sterculia Foetida Linn
© 2019 JETIR May 2019, Volume 6, Issue 5 www.jetir.org (ISSN-2349-5162) PHYTOCHEMICAL SCREENING OF ACTIVE SECONDARY METABOLITES PRESENT IN THE LEAVES EXTRACTS OF STERCULIA FOETIDA LINN. 1Jeyabaskar Suganya, *1Mahendran Radha, 2Rajesh Kumar. Gb 1Assistant Professor, *1Professor, 2 Assistant Professor 1Department of Bioinformatics, 1School of Life Sciences, VISTAS, Pallavaram, Chennai-600117, Tamil Nadu, India. ABSTRACT: Sterculia foetida Linn is a soft handsome woody tree with various pharmacological properties and they are most prevalently found in India, Thailand, Indonesia, Ghana, and Australia. The biochemically active compounds present in the plant possess good medicinal properties which have been already reported in several research papers. The present study was designed to screen the phytochemicals present in the leaf of Sterculia foetida. The leaf powder was successively extracted with polar, non-polar solvents (hexane, chloroform, methanol, ethyl acetate and aqueous) by using various standard protocol for specific secondary metabolite. Secondary metabolites present in the cells of the plant are responsible for medicinal activity of plants. The phytochemical screening of the current study confirms the presence and absence of fifteen secondary metabolites (carbohydrates, tannins, saponin,flavonoids, alkaloids, quinones, terpenoids, glycosides, triterpenoids, phenols, coumarins, proteins, cardiac glycosides, steroids, phytosterols) in the five leaf extracts. The result of the phytochemical profilingfinally revealed that the methanol leaf extract of Sterculia foetida exhibit the presence of high content of important secondary metaboliteswhich possess various pharmacological actionwhen compared with other four leaf extracts. Further, the current research would be helpful for the scientific community to identify and design the new drug molecules with better medicinal activity. Keywords: Sterculia foetida Linn, Leaves, Five solvents, Qualitative analysis, Secondary metabolites. -

Agricultural Marketing Service, USDA § 201.56–10
Agricultural Marketing Service, USDA § 201.56–10 (A) One or more essential structures and become thin, leaf-like, and photo- impaired as a result of decay from pri- synthetic. mary infection. (3) Shoot system: The hypocotyl (B) Albino. elongates carrying the cotyledons above the soil surface. The epicotyl [59 FR 64504, Dec. 14, 1994] usually does not show any development § 201.56–8 Flax family, Linaceae. within the test period. Areas of yel- lowish pigmentation may develop on Kind of seed: Flax. the hypocotyl in cotton. (a) General description. (4) Root system: A primary root, with (1) Germination habit: Epigeal dicot. secondary roots usually developing (Due to the mucilaginous nature of the within the test period. Areas of yel- seed coat, seedlings germinated on lowish pigmentation may develop on blotters may adhere to the blotter and the root in cotton. appear to be negatively geotropic.) (b) Abnormal seedling description. (2) Food reserves: Cotyledons which (1) Cotyledons: expand and become photosynthetic. (i) Less than half of the original cot- (3) Shoot system: The hypocotyl yledon tissue remaining attached. elongates carrying the cotyledons (ii) Less than half of the original cot- above the soil surface. The epicotyl yledon tissue free of necrosis or decay. usually does not show any development (Remove any attached seed coats at within the test period. the end of the test period for evalua- (4) Root system: A primary root, with tion of cotyledons.) secondary roots usually developing (2) Epicotyl: within the test period. (i) Missing. (May be assumed to be (b) Abnormal seedling description. present if both cotyledons are intact.) (1) Cotyledons: (ii) [Reserved] (i) Less than half of the original cot- (3) Hypocotyl: yledon tissue remaining attached. -

Federal Aviation Agency
FEDERAL REGISTER VOLUME 30 • NUMBER 117 Friday, June 18,1965 • Washington, D.C. Pages 7863-7938 Agencies in this issue— Agricultural Research Service Area Redevelopment Administration Atomic Energy Commission Civil Aeronautics Board Civil Service Commission Consumer and Marketing Service Federal Aviation Agency Federal Communications Commission Federal Maritime Commission Federal Trade Commission Food and Drug Administration Interstate Commerce Commission Land Management Bureau Securities and Exchange Commission Detailed list of Contents appears inside. Subscriptions Now Being Accepted S L I P L A W S 89th Congress, 1st Session 1965 Separate prints of Public Laws, published immediately after enactment, with marginal annotations and legislative history references. Subscription Price: $12.00 per Session Published by Office of the Federal Register, National Archives and Records Service, General Services Administration Order from Superintendent of Documents, U.S. Government Printing Office, Washington, D.C.,' 20402 Published dally, Tuesday through Saturday (no publication on Sundays, Monday», or FEDERAL®REGISTER on the day after an official Federal holiday), by the Office of the Federal Register, National Area Code 202 Phone 963-3261 A1®1117®8 a n d R ecords Service, G eneral Services A d m in istra tio n (m ail address Nations _ . , _ _ Archives Building, Washington, D.C. 20408), p u r s u a n t to the authority contained in tne Federal Register Act, approved July 26, 1935 (49 Stat. 500, as amended; 44 U.S.C., ch. 8B), under regulations prescribed by the Admin istrative Committee of the Federal Register, approved by the President (1 CFR Ch. I). Distribution is made only by the Superintendent or Documents, Government Printing Office, Washington, D.C. -

1 Seed Storage, Germination, Quality, and Enhancements
1 Seed Storage, Germination, Quality, and Enhancements Alan G. Taylor School of Integrative Plant Science, Horticulture Unit, Cornell AgriTech, Cornell University, Geneva, New York 14456, USA Before vegetables are harvested, before their The focus of this chapter is on postharvest growth and development, even before the seed- aspects of seeds; the period of seed development ling becomes photosynthetically competent, the and production stages are not addressed. We start source of the vegetable—the seed—holds major with storage of seeds with low moisture content keys to final product yield. To fully comprehend as most vegetable crop seeds have unique char- the complexities of vegetable crop growth and acteristics that permit them to withstand desic- development, we must understand seed physi- cation (Leopold and Vertucci, 1986). Desiccation ology related to storage, germination, quality, tolerance is essential for long-term survival and and enhancements. The challenge is to present allows for a time interval between seed produc- an in-depth knowledge of vegetable seeds that tion and crop production. Most vegetable seeds covers considerable diversity with respect to bo- imbibe water readily and, provided with a suit- tanical classification, seed size, and composition. able environment, will germinate and resume This chapter and book encompasses 33 common active growth. The seed uses its reserve materials vegetable crop seeds from ten plant families following germination and then becomes an (Table 1.1). This diverse group of plants makes a active photosynthetic seedling, fixing its own comprehensive overview of vegetable seeds diffi- carbon and producing energy. cult to achieve, especially since there is little sci- Seed quality is a broad term and encom- entific literature on seed physiology of many passes several attributes of seeds including the small-seeded crops of minor economic import- germination and seedling performance. -

Perennial Edible Fruits of the Tropics: an and Taxonomists Throughout the World Who Have Left Inventory
United States Department of Agriculture Perennial Edible Fruits Agricultural Research Service of the Tropics Agriculture Handbook No. 642 An Inventory t Abstract Acknowledgments Martin, Franklin W., Carl W. Cannpbell, Ruth M. Puberté. We owe first thanks to the botanists, horticulturists 1987 Perennial Edible Fruits of the Tropics: An and taxonomists throughout the world who have left Inventory. U.S. Department of Agriculture, written records of the fruits they encountered. Agriculture Handbook No. 642, 252 p., illus. Second, we thank Richard A. Hamilton, who read and The edible fruits of the Tropics are nnany in number, criticized the major part of the manuscript. His help varied in form, and irregular in distribution. They can be was invaluable. categorized as major or minor. Only about 300 Tropical fruits can be considered great. These are outstanding We also thank the many individuals who read, criti- in one or more of the following: Size, beauty, flavor, and cized, or contributed to various parts of the book. In nutritional value. In contrast are the more than 3,000 alphabetical order, they are Susan Abraham (Indian fruits that can be considered minor, limited severely by fruits), Herbert Barrett (citrus fruits), Jose Calzada one or more defects, such as very small size, poor taste Benza (fruits of Peru), Clarkson (South African fruits), or appeal, limited adaptability, or limited distribution. William 0. Cooper (citrus fruits), Derek Cormack The major fruits are not all well known. Some excellent (arrangements for review in Africa), Milton de Albu- fruits which rival the commercialized greatest are still querque (Brazilian fruits), Enriquito D. -

Extreme Expansion of NBS-Encoding Genes in Rosaceae Yanxiao Jia†, Yang Yuan†, Yanchun Zhang, Sihai Yang* and Xiaohui Zhang*
Jia et al. BMC Genetics (2015) 16:48 DOI 10.1186/s12863-015-0208-x RESEARCH ARTICLE Open Access Extreme expansion of NBS-encoding genes in Rosaceae YanXiao Jia†, Yang Yuan†, Yanchun Zhang, Sihai Yang* and Xiaohui Zhang* Abstract Background: Nucleotide binding site leucine-rich repeats (NBS-LRR) genes encode a large class of disease resistance (R) proteins in plants. Extensive studies have been carried out to identify and investigate NBS-encoding gene families in many important plant species. However, no comprehensive research into NBS-encoding genes in the Rosaceae has been performed. Results: In this study, five whole-genome sequencedRosaceaespecies,includingapple,pear,peach,mei,andstrawberry, were analyzed to investigate the evolutionary pattern of NBS-encoding genes and to compare them to those of three Cucurbitaceae species, cucumber, melon, and watermelon. Considerable differences in the copy number of NBS-encoding genes were observed between Cucurbitaceae and Rosaceae species. In Rosaceae species, a large number and a high proportion of NBS-encoding genes were observed in peach (437, 1.52%), mei (475, 1.51%), strawberry (346, 1.05%) and pear (617, 1.44%), and apple contained a whopping 1303 (2.05%) NBS-encoding genes, which might be the highest number of R-genes in all of these reported diploid plant. However, no more than 100 NBS-encoding genes were identified in Cucurbitaceae. Many more species-specific gene families were classified and detected with the signature of positive selection in Rosaceae species, especially in the apple genome. Conclusions: Taken together, our findings indicate that NBS-encoding genes in Rosaceae, especially in apple, have undergone extreme expansion and rapid adaptive evolution.