Conservation Assessment for the Northern Goshawk in Southeast Alaska
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uncorrected Proof
Policy and Practice UNCORRECTED PROOF BBarney_C011.inddarney_C011.indd 117979 99/13/2008/13/2008 44:11:24:11:24 PPMM UNCORRECTED PROOF BBarney_C011.inddarney_C011.indd 118080 99/13/2008/13/2008 44:11:24:11:24 PPMM Copy edited by Richard Beatty 11 Conservation Values from Falconry Robert E. Kenward Anatrack Ltd and IUCN-SSC European Sustainable Use Specialist Group, Wareham, UK Introduction Falconry is a type of recreational hunting. This chapter considers the conser- vation issues surrounding this practice. It provides a historical background and then discusses how falconry’s role in conservation has developed and how it could grow in the future. Falconry, as defi ned by the International Association for Falconry and Conservation of Birds of Prey (IAF), is the hunting art of taking quarry in its natural state and habitat with birds of prey. Species commonly used for hunt- ing include eagles of the genera Aquila and Hieraëtus, other ‘broad-winged’ members of the Accipitrinae including the more aggressive buzzards and their relatives, ‘short-winged’ hawks of the genus Accipiter and ‘long-winged’ falcons (genus Falco). Falconers occur in more than 60 countries worldwide, mostly in North America, the Middle East, Europe, Central Asia, Japan and southern Africa. Of these countries, 48 are members of the IAF. In the European Union falconry Recreational Hunting, Conservation and Rural Livelihoods: Science and Practice, 1st edition. Edited by B. Dickson, J. Hutton and B. Adams. © 2009 Blackwell Publishing, UNCORRECTEDISBN 978-1-4051-6785-7 (pb) and 978-1-4051-9142-5 (hb). PROOF BBarney_C011.inddarney_C011.indd 118181 99/13/2008/13/2008 44:11:24:11:24 PPMM Copy edited by Richard Beatty 182 ROBERT E. -
Avian Taxonomy
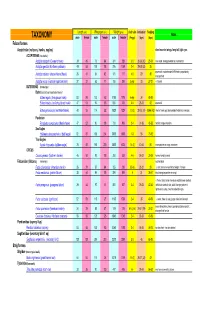
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64 -

Retooling for a Prosperous Ontario
Retooling for a Prosperous Ontario A GLOBAL PERSPECTIVE ON SKILLED TRADES Retooling for a Prosperous Ontario A GLOBAL PERSPECTIVE ON SKILLED TRADES OCTOBER 2006 Table of Contents Executive Summary 3 Introduction 5 SECTION I: ONTARIO 9 Ontario’s Challenges 9 Ontario’s Investment 2 Ontario’s Apprenticeship Program 4 SECTION II: ENVIRONMENTAL SCAN OF OTHER JURISDICTIONS 7 Australia 7 United Kingdom 20 Germany 2 Alberta 26 Manitoba 28 SECTION III: SUMMARY & CONCLUSION 3 APPENDIX I 35 APPENDIX II 37 APPENDIX III 4 RETOOLING FOR A PROSPEROUS ONTARIO Executive Summary This report is an environmental scan of “best practices” from other jurisdictions in Canada and around the world relating to apprenticeship training. The jurisdictions selected in this report represent sources of ideas and strategies that, given a thorough analysis and evaluation, could be successfully applied in Ontario. Ontario has several outstanding programs in place to help address the skilled trades shortage. However, individuals are often unaware of the programs and incentives that currently exist in Ontario nor are they aware of how to access information on such programs. Our research indicates that the current challenges Ontario faces in regards to apprenticeship training and the skilled labour shortage are similar to those experienced in many other jurisdictions. A few examples of these challenges are: . a negative perception associated with a career in a skilled trade; . a lack of awareness; . a hesitation from employers to train an apprentice due to training costs and “poaching”. When reviewing “best practices” in other jurisdictions, several themes are evident when analyzing these successful ideas and strategies. These “themes” can be broken down into the following: . -

Compendium of Avian Ecology
Compendium of Avian Ecology ZOL 360 Brian M. Napoletano All images taken from the USGS Patuxent Wildlife Research Center. http://www.mbr-pwrc.usgs.gov/id/framlst/infocenter.html Taxonomic information based on the A.O.U. Check List of North American Birds, 7th Edition, 1998. Ecological Information obtained from multiple sources, including The Sibley Guide to Birds, Stokes Field Guide to Birds. Nest and other images scanned from the ZOL 360 Coursepack. Neither the images nor the information herein be copied or reproduced for commercial purposes without the prior consent of the original copyright holders. Full Species Names Common Loon Wood Duck Gaviiformes Anseriformes Gaviidae Anatidae Gavia immer Anatinae Anatini Horned Grebe Aix sponsa Podicipediformes Mallard Podicipedidae Anseriformes Podiceps auritus Anatidae Double-crested Cormorant Anatinae Pelecaniformes Anatini Phalacrocoracidae Anas platyrhynchos Phalacrocorax auritus Blue-Winged Teal Anseriformes Tundra Swan Anatidae Anseriformes Anatinae Anserinae Anatini Cygnini Anas discors Cygnus columbianus Canvasback Anseriformes Snow Goose Anatidae Anseriformes Anatinae Anserinae Aythyini Anserini Aythya valisineria Chen caerulescens Common Goldeneye Canada Goose Anseriformes Anseriformes Anatidae Anserinae Anatinae Anserini Aythyini Branta canadensis Bucephala clangula Red-Breasted Merganser Caspian Tern Anseriformes Charadriiformes Anatidae Scolopaci Anatinae Laridae Aythyini Sterninae Mergus serrator Sterna caspia Hooded Merganser Anseriformes Black Tern Anatidae Charadriiformes Anatinae -

Information on Haliaeetus Leucocephalus
ADW: Haliaeetus leucocephalus: Information Page I of 5 University of Michigan Museum of Zoology Structured Inquiry Searcl Home > Kingdom Animalia >- Phylum Chordata U Subphylum Vertebrata ,> Class Aves1 Order Falconiformes t- Family Accipitridae P Subfamily Accipitrinae r Species Haliaeetus leucocephalus Haliaeetusleucocephalus bald eagle Information Pictures I4-0Classification I 2008/01/20 04:31:08.826 US/Eastern By Marie S. Harris Geographic Range Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Aves Order: Falconiformes Family: Accipitridae Subfamily: Accipitrinae Genus: Haliaeetus Species: Haliaeetus leucocephallus The bald eagle is native to North America and originally bred from central Alaska and northern Canada south to Baja California, central Arizona, and the Gulf of Mexico. It now has been extirpated in many southern areas of this range. Biogeographic Regions: nearctic CL (native (k). Habitat Bald eagles are able to live anywhere on the North American continent where there are adequate nest trees, roosts ands feeding grounds. Open water such as a lake or an ocean, however, is a necessity. Terrestrial Biomnes: desert or dune Ck; savanna or grassland Q,; chaparral Q,; forest Q.; mountains Q. http://animaldiversity.ummz.umich.edu/site/accounts/information/Haliaeetus-leucoceph... 1/23/2008 ADW: Haliaeetus leucocephalus: Information Page 2 of 5 Physical Description Mass 3175 g (average) [Ref] (111.76 oz) The plumage of an adult bald eagle is brown with a white head and tail. Immature eagles are irregularly mottled with white until the fourth year. Their legs are feathered half way down the tarsus, and the beak, feet, and eyes are bright yellow. Bald eagles have massive tarsi, short and powerful grasping toes, and long talons. -

THE RETAIL SALES TAX: an ECONOMIC STUDY by KENYON E
THE RETAIL SALES TAX: AN ECONOMIC STUDY by KENYON E. POOLE A study prepared for The Ontario Committee on Taxation THE RETAIL SALES TAX: AN ECONOMIC STUDY by KENYON E. POOLE, Ph.D. A study prepared for The Ontario Committee on Taxation Printed and Published by FRANK FOGG Queen’s Printer Preface TH E present study was submitted at the beginning of 1964. Much has happened ■ since then with respect to all systems of taxation, including those of the Canadian provinces and the American states. Time limitations have prevented bringing these data up to 1968. The author would like, moreover, to take this opportunity to emphasize that his favourable view with respect to the sales tax is based on two considerations. First, the sales tax is only one tax in the revenue system as a whole. Second, exemptions can be provided for food, clothing, etc., which make the tax at least proportional in the first several thousand dollars of income and spending. In a recommendation in favour of a sales tax there is no implication that any desired use may not be made of a progressive income tax, a graduated inheritance tax, or even a graduated net worth tax. The several taxes can be combined in such a way as to satisfy the public’s view on the desired after-tax distribution of income and wealth. The author’s thanks are gladly given to those who contributed to the study at key points along the line of its development Robert Evans is due special thanks for preparing the first two chapters. -

Proposals 2018-C
AOS Classification Committee – North and Middle America Proposal Set 2018-C 1 March 2018 No. Page Title 01 02 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae 02 10 Split Yellow Warbler (Setophaga petechia) into two species 03 25 Revise the classification and linear sequence of the Tyrannoidea (with amendment) 04 39 Split Cory's Shearwater (Calonectris diomedea) into two species 05 42 Split Puffinus boydi from Audubon’s Shearwater P. lherminieri 06 48 (a) Split extralimital Gracula indica from Hill Myna G. religiosa and (b) move G. religiosa from the main list to Appendix 1 07 51 Split Melozone occipitalis from White-eared Ground-Sparrow M. leucotis 08 61 Split White-collared Seedeater (Sporophila torqueola) into two species (with amendment) 09 72 Lump Taiga Bean-Goose Anser fabalis and Tundra Bean-Goose A. serrirostris 10 78 Recognize Mexican Duck Anas diazi as a species 11 87 Transfer Loxigilla portoricensis and L. violacea to Melopyrrha 12 90 Split Gray Nightjar Caprimulgus indicus into three species, recognizing (a) C. jotaka and (b) C. phalaena 13 93 Split Barn Owl (Tyto alba) into three species 14 99 Split LeConte’s Thrasher (Toxostoma lecontei) into two species 15 105 Revise generic assignments of New World “grassland” sparrows 1 2018-C-1 N&MA Classification Committee pp. 87-105 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae Background: Our current linear sequence of the Accipitridae, which places all the kites at the beginning, followed by the harpy and sea eagles, accipiters and harriers, buteonines, and finally the booted eagles, follows the revised Peters classification of the group (Stresemann and Amadon 1979). -

The Association of Professional Archaeologists Newsletter
The Association of Professional Archaeologists Newsletter 2013-02 WINTER EDITION Web Page: www.apaontario.ca Facebook: www.facebookcom/APAOntario Your New Executive Directors Archaeological Society in 2007. She has worked with • President, Sue Bazely numerous heritage organizations and has identified • Vice President, Keith Powers broadening the scope of representation as her key aim • Past President, Scarlett Janusas for the APA. • Director, Membership and Webmaster, Keith Powers has conducted archaeological fieldwork James B Bandow in Ontario for over 15 years. He received formal • Director, First Nations, Laurie Jackson training in archaeological fieldwork through the Boyd • Director, Northern Ontario, Scott Archaeological Field School at the Seed-Barker site. Hamilton Keith received academic training in archaeology • Director, Investigations, Carla Parslow through York University and the University of Toronto. • Director, Field Director, Jeff Muir Keith completed his graduate work at the University of • Director, Innovations and Process, York, England where his research focused on the Douglas Yahn application of geophysical techniques to Iroquoian archaeology. Other Members Since 2002 he has been the Principal Archaeologist of • Treasurer, Cathy Crinnion The Archaeologists Inc. and has conducted hundreds of archaeological assessments within the province. He • Secretary , Open continues to pursue research interests in the use of • Newsletter, Tom Arnold geophysical techniques in archaeological investigations and has applied these techniques to a variety of sites Executive Bios including Iroquoian villages, historic homesteads, and cemeteries. Sue Bazely has an MA in Archaeology and Heritage from the University of Leicester (UK) and BA in Keith has worked on archaeological projects Anthropology (Archaeology) from the University of throughout Canada and worldwide including sites in Toronto. -

University of Cape Town
Exploring the breeding diet of the Black Sparrowhawk (Accipiter Melanoleucus) on the Cape Peninsula Honours research project by Bruce Baigrie Biological Sciences Department: University of Cape Town Supervised by Dr. Arjun Amar Town Cape of University Page 1 of 33 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University Abstract This study investigates the diet of breeding Black Sparrowhawks (Accipiter melanoleucus) on the Cape Peninsula of South Africa. Macro-remains of prey were collected from below and around the vicinity of nests throughout the breeding seasons of 2012 and 2013. These prey items were then identified down to species where possible through the use of a museum reference collection. In both years 85.9% of the individual remains were those of Columbidae, which corresponds with the only other diet study on Black Sparrowhawks. Red- eyed Doves were the most common prey species, accounting for around 35% of the diet’s biomass and 45% of the prey items. Helmeted Guineafowl were also an important component of the diet for certain nests, making up on average 26.4% biomass of the diet. I found very little difference in diet between the different stages of breeding (pre-lay, incubation and nestling), despite the fact that females only contribute significantly during the nestling state and are considerably larger than the males. -

The Systematic Position of Certain Hawks in the Genus Buteo
THE AUK A QUARTERLY JOURNAL OF ORNITHOLOGY VoL. 80 OCTOnER,1963 No. 4 THE SYSTEMATIC POSITION OF CERTAIN HAWKS IN THE GENUS BUTEO N•.v K. Jo•so• A•V I•s J. P•.•.T•.RS THE mostrecent authoritative work dealingwith the North and Middle AmericanFalconiformes states that the genusButeo "is sucha composite (that is, is so variablewithin its includedsubgenera) that any description of the genusas a wholewould be largelya matter of exceptionsin one or anothersubgenus of all the genericcharacters." In otherwords, the group "defiessubdivision" (Friedmann, 1950: 212-213), and it is apparentthat the existingsubgeneric classification of North Americanforms is largely subjective. The presentreport attempts to delineatewhat seemsto be a natural unit of five New World speciesof Buteo basedupon certaincommon fea- turesof plumage,proportions, distribution, and behavior.These species, hereafter referred to as the "woodlandbuteos," are the RoadsideHawk ( B. magnirostris) , Ridgway'sHawk ( B. ridgwayi) , Red-shoulderedHawk (B. lineatus),Broad-winged Hawk (B. platypterus),and Gray Hawk or Mexican Goshawk (B. nitidus). GEOG•Ar•IC A•I) ECOLOGIC D•ST•mUT•O• For purposesof discussion,the followingsynopsis of distributionof the woodlandbuteos is presented.Except whereotherwise noted, statements regardinggeographic range are basedon the Check-listof North American birds (A.O.U., 1957) and on the Distributionalcheck-list of the birds of Mexico, Part I (Friedmann et al., 1950). The Roadside Hawk occurs from Mexico to Argentina, with 16 races recognizedby Hellmayrand Conover(1949) and alsoby Peters(1931). Mexicanraces prefer open country, second-growth woods, and forestedge (Blake, 1953). In the provinceof Bocasdel Toro, Panama,Eisenmann (1957: 250) foundit to be an "edge"species. -

Accipitridae Species Tree
Accipitridae I: Hawks, Kites, Eagles Pearl Kite, Gampsonyx swainsonii ?Scissor-tailed Kite, Chelictinia riocourii Elaninae Black-winged Kite, Elanus caeruleus ?Black-shouldered Kite, Elanus axillaris ?Letter-winged Kite, Elanus scriptus White-tailed Kite, Elanus leucurus African Harrier-Hawk, Polyboroides typus ?Madagascan Harrier-Hawk, Polyboroides radiatus Gypaetinae Palm-nut Vulture, Gypohierax angolensis Egyptian Vulture, Neophron percnopterus Bearded Vulture / Lammergeier, Gypaetus barbatus Madagascan Serpent-Eagle, Eutriorchis astur Hook-billed Kite, Chondrohierax uncinatus Gray-headed Kite, Leptodon cayanensis ?White-collared Kite, Leptodon forbesi Swallow-tailed Kite, Elanoides forficatus European Honey-Buzzard, Pernis apivorus Perninae Philippine Honey-Buzzard, Pernis steerei Oriental Honey-Buzzard / Crested Honey-Buzzard, Pernis ptilorhynchus Barred Honey-Buzzard, Pernis celebensis Black-breasted Buzzard, Hamirostra melanosternon Square-tailed Kite, Lophoictinia isura Long-tailed Honey-Buzzard, Henicopernis longicauda Black Honey-Buzzard, Henicopernis infuscatus ?Black Baza, Aviceda leuphotes ?African Cuckoo-Hawk, Aviceda cuculoides ?Madagascan Cuckoo-Hawk, Aviceda madagascariensis ?Jerdon’s Baza, Aviceda jerdoni Pacific Baza, Aviceda subcristata Red-headed Vulture, Sarcogyps calvus White-headed Vulture, Trigonoceps occipitalis Cinereous Vulture, Aegypius monachus Lappet-faced Vulture, Torgos tracheliotos Gypinae Hooded Vulture, Necrosyrtes monachus White-backed Vulture, Gyps africanus White-rumped Vulture, Gyps bengalensis Himalayan -

Phylogeny, Historical Biogeography and the Evolution of Migration in Accipitrid Birds of Prey (Aves: Accipitriformes)
Ornis Hungarica 2014. 22(1): 15–35. DOI: 10.2478/orhu-2014-0008 Phylogeny, historical biogeography and the evolution of migration in accipitrid birds of prey (Aves: Accipitriformes) Jenő nagy1 & Jácint tökölyi2* Jenő Nagy & Jácint Tökölyi 2014. Phylogeny, historical biogeography and the evolution of mig ration in accipitrid birds of prey (Aves: Accipitriformes). – Ornis Hungarica 22(1): 15–35. Abstract Migration plays a fundamental part in the life of most temperate bird species. The re gu lar, largescale seasonal movements that characterize temperate migration systems appear to have originated in parallel with the postglacial northern expansion of tropical species. Migratoriness is also in- fluenced by a number of ecological factors, such as the ability to survive harsh winters. Hence, understanding the origins and evolution of migration requires integration of the biogeographic history and ecology of birds in a phylogenetic context. We used molecular dating and ancestral state reconstruction to infer the origins and evolu- tionary changes in migratory behavior and ancestral area reconstruction to investigate historical patterns of range evolution in accipitrid birds of prey (Accipitriformes). Migration evolved multiple times in birds of prey, the ear- liest of which occurred in true hawks (Accipitrinae), during the middle Miocene period, according to our analy- ses. In most cases, a tropical ancestral distribution was inferred for the nonmigratory ancestors of migratory line- ages. Results from directional evolutionary tests indicate that migration evolved in the tropics and then increased the rate of colonization of temperate habitats, suggesting that temperate species might be descendants of tropi- cal ones that dispersed into these seasonal habitats.