Finding Niebla Homalea: Predicting the Distribution of a Fog Lichen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

S1 the Three Areas of Importance for the Fruticose Ramalinaceae.FINAL
Supplementary Data S1 Three Major Ecogeographic Areas of Evolution in Fruticose Ramalinaceae Three geographical areas appear significant to the evolutionary history of lichens in arid regions. We focus on three fruticose genera of the Ramalinaceae: Nambialina (gen. nov.), Niebla, and Vermilacinia. They have adapted to obtaining moisture from fog in the following coastal regions of our study: (I) California-Baja California coastal chaparral and Vizcaíno deserts (II) South America Atacama and Sechura deserts (III) South Africa coastal fynbos and Namib Desert The botanical significance of each of these will be briefly discussed. Chapter (I) further includes updates on the ecogeographical data and evolutionary interpretation for the genera Niebla and Vermilacinia in Baja California. (I) California and Baja California Coastal Chaparral, Chaparral-Desert Transition and Vizcaíno Deserts 1. California and Baja California Coastal Chaparral The lichen flora of the Baja California peninsula (Mexico, Baja California and Baja California Sur) is pretty well known as a result of the many splendid contributions by lichenologists to the « Lichen Flora of the Greater Sonoran Desert Region » (Nash et al. 2002, 2004, 2007). The « Greater » portion extends the lichen flora to chaparral, oak woodlands, and conifer forests in southern California, northern Baja California and southern Nevada and all of Arizona; to the deserts of Mojave, Arizona, Vizcaíno, and Magdalena; and to the subtropical vegetation in Baja California Sur and Sonora, Mexico that include lowland mixed deciduous-succulent bushlands, open deciduous montane woodlands/forests, and the evergreen pine-oak woodlands/forests. California, a botanically rich and diverse region with many endemic species (Raven and Axelrod 1978 ; Calsbeek et al. -

Niebla Ramosissima
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2020: T175709793A175710677 Scope(s): Global Language: English Niebla ramosissima Assessment by: Reese Næsborg, R. View on www.iucnredlist.org Citation: Reese Næsborg, R. 2020. Niebla ramosissima. The IUCN Red List of Threatened Species 2020: e.T175709793A175710677. https://dx.doi.org/10.2305/IUCN.UK.2020- 3.RLTS.T175709793A175710677.en Copyright: © 2020 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Fungi Ascomycota Lecanoromycetes Lecanorales Ramalinaceae Scientific Name: Niebla ramosissima Spjut Assessment Information Red List Category & Criteria: Vulnerable D2 ver 3.1 Year Published: 2020 Date Assessed: July 9, 2020 Justification: Niebla ramosissima is narrowly endemic to San Nicolas Island where it is known from one location and its Area of Occupancy = 16 km2 (up to a maximum of 32 km2). -

Sexual Reproductive Characters Vs
J. Hattori Bot. Lab. No. 76: 207-219 (Oct. 1994) SEXUAL REPRODUCTIVE CHARACTERS VS. MORPHOLOGICAL CHARACTERS IN LICHEN GENERA E. I. KA.RNEFELT1 AND ARNE THELL2 ABSTRACT. The generic concept and its early historical development until today's more natural multi-character based concept is discussed. The modern generic concept for lichenized ascomy cetes has traditionally been slightly different concerning crustose groups on the one hand and foliose and fruticose groups on the other. More studies of sexual reproductive st ructures in the large groups of lichens should presumably be carried out since it has been demonstrated recently that a considerable variation actually occurs in both asci and pycnidia in the cetrarioid genera. Some examples of difficult cases of evaluation of structural characters in relation to characters in the sexual reproductive structures in cetrarioid genera and the Teloschistaceae are also discussed. INTRODUCTION Almost two centuries ago during the classical period of botanical history, genera were basically described on simply recognized morphological characters or in a combination of the position of apothecia and pycnidia. Acharius ( 1803) in his Metho dus Lichenum separated 39 genera of which some are still valid, though in a different way, such as Alectoria, Cetraria, Evernia, Lecanora, Lecidea, Nephroma, Parmelia and Ramalina. Apothecial structures were also used later at the end of the classical period for the understanding of generic concepts. It was, however, not until in 1852 that spore characteristics were introduced by Massalongo for the recognition of genera. Massa longo even ranked characters in a certain order of importance, such as spores, asci, paraphyses, hypothecium, exciple and finally general thallus characters (Massalongo 1852). -

Bulletin of the California Lichen Society
Bulletin of the California Lichen Society Volume 2 No. 1 Spring 1995 The California Lichen Society seeks to promote the appreciation, conservation, and study of the lichens. The focus of the Society is on California, but its interests include the entire western part of the continent. Dues are $10 per year payable to The California Lichen Society, 1200 Brickyard Way, #302, Point Richmond, CA 94801. Members receive the Bulletin and notices of meetings, field trips, and workshops. The Bulletin of the California Lichen Society is edited by Isabelle Tavares and Darrell Wright and is produced by Darrell Wright and Bill Hill. The Bulletin welcomes manuscripts on technical topics in lichenology relating to western North America and on conservation of the lichens, as well as news of lichenologists and their activities. The best way to submit manuscripts apart from short articles and announcements is on 1 .2 or 1.44 Mb diskette in Word Perfect 4.1, 4 .2 or 5.1 format; ASCII format is an alternative. A review process is followed, and typed manuscripts should be double-spaced and submitted as two copies. Figures are the usual line drawings and sharp black and white glossy photos, unmounted. Nomenclature follows Egan's Fifth Checklist and supplements (Egan 1 987, 1989, 1990: this bibliography is in the article on the Sonoma Mendocino County field trip, Bull. Cal. Lich . Soc. 1 (2):3) Style follows this issue. Reprints will be provided for a nominal charge. Address submittals and correspondence to The California Lichen Society, c/o Darrell Wright, 2337 Prince Street, Berkeley, CA 94705, 510-644-8220, voice and FAX (new capability); E-mail: [email protected] (new address). -

Constancea 85: Tucker, Catalog of California Lichens
Constancea 85, 2014 University and Jepson Herbaria California Lichen Catalog CATALOG OF LICHENS, LICHENICOLES AND ALLIED FUNGI IN CALIFORNIA (second revision) Shirley C. Tucker Cheadle Center for Biodiversity, Department of Ecology, Evolution & Marine Biology, University of California, Santa Barbara, CA 93106–9610 USA; Santa Barbara Botanic Garden, Santa Barbara, CA; and Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803 [email protected] Type all or part of a lichen name into the search box. search ABSTRACT This second revision of the California lichen catalog reports 1,869 taxa at species level and below (of which 58 are recognized at the level of variety, subspecies, or forma) for the state, an increase of about 295 taxa since 2006, and 565 taxa since the 1979 catalog. The number of genera is 340, an increase of 43 since 2006. The lichen flora of California includes about 35% of the 5,246 species and 52% of the 646 genera reported for the continental United States and Canada. All known references are given that cite each species as occurring in California. Synonyms are cross-referenced to the current names. Accepted names are listed first, followed by names of taxa that are either excluded or not confirmed. The bibliography includes 1158 publications pertaining to California lichens (citations through 2011, and some from 2012). Key words: California; Flora; Lichens; Lichenicoles The 34 years since publication of the first catalog of California lichens (Tucker & Jordan 1979) have seen enormous changes in accepted names of lichens, particularly an increase in generic names. The Tucker & Jordan catalog was originally based on literature records assembled by William A. -

All BLM CALIFORNIA SPECIAL STATUS PLANTS
All BLM CALIFORNIA SPECIAL STATUS PLANTS Thursday, May 28, 2015 11:00:38 AM CA RARE PLANT RANK RECOVERY PLAN? PALM SPRINGS MOTHER LODE GLOBAL RANK NNPS STATUSNNPS BAKERSFIELD BLM STATUS RIDGECREST STATE RANK FED STATUS EAGLE LAKE NV STATUS EL CENTRO CA STATUS HOLLISTER TYPE BARSTOW SURPRISE REDDING ALTURAS NEEDLES ARCATA OF DATE BISHOP SCIENTIFIC NAME COMMON NAME PLANT FAMILY UPDATED COMMENTS UKIAH Abronia umbellata var. pink sand-verbena VASC Nyctaginaceae BLMS 1B.1 G4G5T2 S1 No 29-Apr-13 Formerly subsp. breviflora (Standl.) K breviflora Munz. Abronia villosa var. aurita chaparral sand-verbena VASC Nyctaginaceae BLMS 1B.1 G5T3T4 S2 No 06-Aug-13 CNDDB occurrences 2 and 91 are on S K BLM lands in the Palm Springs Field Office. Acanthomintha ilicifolia San Diego thornmint VASC Lamiaceae FT SE 1B.1 G1 S2 No 12-Mar-15 Status changed from "K" to "S" on S 8/6/2013. Naomi Fraga was unable to find the species on BLM lands when trying to collect seeds in 2012. Although there are several CNDDB occurences close to BLM lands, none of these actually intersect with BLM lands. Acanthoscyphus parishii Cushenberry oxytheca VASC Polygonaceae FE 1B.1 G4?T1 S1 No 06-Aug-13 Formerly Oxytheca parishii var. K var. goodmaniana goodmaniana. Name change based on Reveal, J.L. 2004. Nomenclatural summary of Polygonaceae subfamily Eriogonoideae. Harvard Papers in Botany 9(1):144. A draft Recovery Plan was issued in 1997 but as of 8/6/2013 was not final. Some of the recovery actions in the draft plan have been started and partially implemented. -

Channel Islands Checklist of Lichens
Opuscula Philolichenum, 11: 145-302. 2012. *pdf effectively published online 23October2012 via (http://sweetgum.nybg.org/philolichenum/) The Annotated Checklist of Lichens, Lichenicolous and Allied Fungi of Channel Islands National Park 1 2 KERRY KNUDSEN AND JANA KOCOURKOVÁ ABSTRACT. – For Channel Islands National Park, at the beginning of the 21st century, a preliminary baseline of diversity of 504 taxa in 152 genera and 56 families is established, comprising 448 lichens, 48 lichenicolous fungi, and 8 allied fungi. Verrucaria othmarii K. Knudsen & L. Arcadia nom. nov. is proposed for the illegitimate name V. rupicola (B. de Lesd.) Breuss, concurrently a neotype is also designated. Placidium boccanum is reported new for North America and California. Bacidia coprodes and Polycoccum pulvinatum are reported new for California. Catillaria subviridis is verified as occurring in California. Acarospora rhabarbarina is no longer recognized as occurring in North America. Seven species are considered endemic to Channel Islands National Park: Arthonia madreana, Caloplaca obamae, Dacampia lecaniae, Lecania caloplacicola, Lecania ryaniana, Plectocarpon nashii and Verrucaria aspecta. At least 54 species, many of which occur in Mexico, are only known in California from Channel Islands National Park INTRODUCTION Channel Islands National Park (heretofore referred to as CINP) in Ventura and Santa Barbara Counties in southern California consists of five islands totalling approximately 346 square miles in area (221331 acres, 89569 ha, 900 km2). Santa Barabara Island, with an area of 1.02 square miles (639 acres, 259 ha, 2.63 km2) is the smallest island in CINP. It is a considered a member of the southern islands which include San Nicolas Island, Santa Catalina Island, and San Clemente Island, all outside the boundaries of the national park. -

Species Boundaries in the Messy Middle
Species boundaries in the messy middle – testing the hypothesis of micro-endemism in a recently diverged lineage of coastal fog desert lichen fungi Jesse Jorna1, Jackson Linde1, Peter Searle1, Abigail Jackson1, Mary-Elise Nielsen1, Madeleine Nate1, Natalie Saxton1, Felix Grewe2, Mar´ıade los Angeles Herrera-Campos3, Richard Spjut4, Huini Wu2, Brian Ho2, Steven Leavitt1, and Thorsten Lumbsch2 1Brigham Young University 2Field Museum of Natural History 3Universidad Nacional Aut´onomade M´exicoInstituto de Biolog´ıa 4World Botanical Associates April 5, 2021 Abstract Species delimitation among closely related species is challenging because traditional phenotype-based approaches, e.g., morphol- ogy, ecological, or chemical characteristics, often produce conflicting results. With the advent of high-throughput sequencing, it has become increasingly cost-effective to acquire genome-scale data which can resolve previously ambiguous species boundaries. As the availability of genome-scale data has increased, numerous species delimitation analyses, such as BPP and SNAPP+Bayes factor delimitation (BFD*), have been developed to delimit species boundaries. However, even empirical molecular species delimitation approaches can be biased by confounding evolutionary factors, e.g., hybridization/introgression and incomplete lineage sorting, and computational limitations. Here we investigate species boundaries and the potential for micro-endemism in a lineage of lichen-forming fungi, Niebla Rundel & Bowler in the family Ramalinaceae. The species delimitation models tend to support more specious groupings, but were unable to infer robust, consistent species delimitations. The results of our study highlight the problem of delimiting species, particularly in groups such as Niebla, with complex, recent phylogeographic histories. Species boundaries in the messy middle – testing the hypothesis of micro-endemism in a recently diverged lineage of coastal fog desert lichen fungi Jesse Jorna1,*, Jackson B. -

Survey Protocols for Lichens
SURVEY PROTOCOLS FOR CATEGORY A & C LICHENS Bryoria tortuosa (G. Merr.) Brodo & D. Hawksw. Leptogium cyanescens (Rabenh.) Körber Lobaria oregana (Tuck.) Müll. Niebla cephalota (Tuck.) Rundel & Bowler Platismatia lacunosa (Ach.) Culb. & C. Culb. Ramalina thrausta (Ach.) Nyl. Teloschistes flavicans (Sw.) Norman Usnea longissima Ach. Usnea longissima Ach. Version 2.0 September 2002 By: Chiska Derr, Richard Helliwell, Andrea Ruchty, Lisa Hoover, Linda Geiser, David Lebo & John Davis U.S. DEPARTMENT OF THE INTERIOR BUREAU OF LAND MANAGEMENT BLM/OR/WA/PL-02/045+1792 All photographs used in this document are copyrighted © by Sylvia and Stephen Sharnoff and are used with their permission. TABLE OF CONTENTS INTRODUCTION...................................................................................................... 5 SECTION I: SURVEY PROTOCOLS ...................................................................... 5 I. SURVEY METHODS.......................................................................................... 5 A. Pre-field Review/Trigger for Survey......................................................... 5 B. Field Survey .................................................................................................. 6 C. Extent of Surveys .......................................................................................... 7 D. Timing of Surveys ........................................................................................ 8 E. Determination of Habitat Disturbing Activities with Significant Negative Effects -

Manual for Monitoring Air Quality Using Lichens on National Forests of the Pacific Northwest
United States Department of Agriculture Manual for Monitoring Air Forest Service Quality Using Lichens on Pacific Northwest Region National Forests of the Pacific Air Resource Management Northwest R6-NR-ARM-TP-02-04 Field protocols Laboratory protocols Individualized sampling strategies for nine national forests Manual for Monitoring Air Quality Using Lichens on National Forests of the Pacific Northwest Field protocols, laboratory protocols, and individual sampling strategies for the Columbia River Gorge National Scenic Area Deschutes National Forest Gifford Pinchot National Forest Mt. Hood National Forest Siuslaw National Forest Umpqua National Forest Wallowa-Whitman National Forest Willamette National Forest and Winema National Forest US Department of Agriculture-Forest Service, Pacific Northwest Region, Air Resource Management, Portland, Oregon January 2004 Cover illustration of Nephroma laevigatum by Alexander Mikulin. Copies of this document can be obtained from: Program Director, Air Resource Management, USDA-Forest Service Pacific Northwest Region, 333 SW 1st Street, Portland, OR 97204. This document is also available on-line at the url < http://www.fs.fed.us/r6/aq >. Suggested citation: Geiser, L. 2004. Monitoring Air Quality Using Lichens on National Forests of the Pacific Northwest: Methods and Strategy. USDA-Forest Service Pacific Northwest Region Technical Paper, R6-NR-AQ-TP-1-04. 134 pp. The U. S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activates on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, and marital or family status. Persons with disabilities who require alternative means for communication of program information should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). -
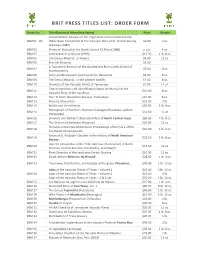
Brit Press Titles List: Order Form
BRIT PRESS TITLES LIST: ORDER FORM Series No. Title (Botanical Miscellany Series) Price Weight A Quantitative Analysis of the Vegetation on the Dallas County SBM 01 - 02 White Rock Escarpment & The Vascular Flora of St. Francis County, $3.00 4 oz. Arkansas (1987) SBM 03 Shinners’ Manual of the North Central TX Flora (1988) o-o-p 4 oz. SBM 04 Asteraceae of Louisiana (1989) $12.50 1 lb. 8 oz. SBM 05 The Genus Mikania…in Mexico $4.00 12 oz. SBM 06 Frontier Botanist… $5.00 A Taxonomic Revision of the Acaulescent Blue Violets (Viola) of SBM 07 $5.00 8 oz. North America SBM 08 Aster and Brachyactis (Asteraceae) in Oklahoma $5.00 8 oz. SBM 09 The Genus Mikania…in the Greater Antilles $7.00 8 oz. SBM 10 Checklist of the Vascular Plants of Tennessee $7.00 12 oz. Text Annotations and Identification Notes for Manual of the SBM 11 $10.00 8 oz. Vascular Flora of the Carolinas SBM 12 The “El Cielo” Biosphere Reserve, Tamaulipas $10.00 8 oz. SBM 13 Flora de Manantlán $22.50 2 lb. SBM 14 Niebla and Vermilacinia… $25.00 1 lb. 4 oz. Monograph of Northern Mexican Crataegus (Rosaceae, subfam. SBM 15 $16.50 12 oz. Maloideae) SBM 16 Shinners and Mahler’s Illustrated Flora of North Central Texas $89.95 7 lb. 8 oz. SBM 17 The Grasses of Barbados (Poaceae) $10.00 12 oz. Floristics in the New Millennium: Proceedings of the Flora of the SBM 18 $10.00 1 lb. 4 oz. Southeast US Symposium Emanuel D. -
The Lichen of British Columbia
LICHINODIUM Nyl. The Bearhair Lichens Minute nonstratified fruticose (shrub) globose, colourless, to eight per ascus. lichens, consisting of rosettes of decum- Over rock-dwelling lichens and bark of bent to semi-erect branches, these black- conifers. ish (translucent when moist), round in cross-section, slender, to .‒. (–.) References: Henssen (1963, 1968); mm long and 40–80 (–150) µm wide, dull, Arvidsson (1979). noncorticate, brittle, solid, rather sparsely Common Name: Fanciful. branched; branching irregular. Soredia, Notes: Lichinodium is a primarily temper- isidia, pseudocyphellae, and medulla ate genus consisting of four species absent. Attached to substrate by colourless worldwide. Three of these have been hairlike rhizoids. Photobiont cyanobacter- reported from North America, and two ial, Scytonema, cells in two to several from British Columbia. No chemical rows, to 9–15 (–17) µm along long axis. substances have been reported. Ascocarp an apothecium, borne Two keys are provided: the first is to laterally, unknown in local material, Lichinodium, and the second is to disc pale brown, to 0.2–0.8 mm across. L. canadense and similar small, black- Spores 1-celled, ellipsoid to nearly ish, tree-dwelling fruticose lichens. KEY TO LICHINODIUM 1a Over conifer bark in humid old-growth forests; branches to 0.4–0.8 mm long . Lichinodium canadense 1b Over rock-dwelling lichens (and mosses), apparently restricted to nonforested sites; branches to 1–2 mm long . Lichinodium sirosiphoideum KEY TO LICHINODIUM CANADENSE AND SIMILAR SMALL BLACKISH FRUTICOSE LICHENS OCCURRING OVER TREES 1a Conspicuous, to more than 7.0 mm long; medulla present, white, usual- 2a ly distinct; photobiont 1-celled (check under LM); soredia present or x8 absent; distribution various .