Distribution, Habitat Structure and Troop Size in Eastern Cape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biodiversity and Ecology of Afromontane Rainforests with Wild Coffea Arabica L
Feyera Senbeta Wakjira (Autor) Biodiversity and ecology of Afromontane rainforests with wild Coffea arabica L. populations in Ethiopia https://cuvillier.de/de/shop/publications/2260 Copyright: Cuvillier Verlag, Inhaberin Annette Jentzsch-Cuvillier, Nonnenstieg 8, 37075 Göttingen, Germany Telefon: +49 (0)551 54724-0, E-Mail: [email protected], Website: https://cuvillier.de General introduction 1 GENERAL INTRODUCTION 1.1 Background The term biodiversity is used to convey the total number, variety and variability of living organisms and the ecological complexes in which they occur (Wilson 1988; CBD 1992; Rosenzweig 1995). The concept of biological diversity can be applied to a wide range of spatial and organization scales, including genetics, species, community, and landscape scales (Noss 1990; Austin et al. 1996; Tuomisto et al. 2003). It is becoming increasingly apparent that knowledge of the role of patterns and processes that determine diversity at different scales is at the very heart of an understanding of variation in biodiversity. Processes influencing diversity operate at different spatial and temporal scales (Rosenzweig 1995; Gaston 2000). A variety of environmental events and processes, including past evolutionary development, biogeographic processes, extinctions, and current influences govern the biodiversity of a particular site (Brown and Lomolino 1998; Gaston 2000; Ricklefs and Miller 2000). Biodiversity is valued and has been studied largely because it is used, and could be used better, to sustain and improve human well-being (WWF 1993; WCMC 1994). However, there has been a rapid decline in the biodiversity of the world during the past two to three decades (Wilson 1988; Whitmore and Sayer 1992; Lugo et al. -

Herpetofaunal Survey of the Ongeluksnek (Malekgalonyane) Nature Reserve in the Foothills of the Drakensberg, Eastern Cape Province, South Africa
Herpetology Notes, volume 13: 717-730 (2020) (published online on 25 August 2020) Herpetofaunal survey of the Ongeluksnek (Malekgalonyane) Nature Reserve in the foothills of the Drakensberg, Eastern Cape Province, South Africa Werner Conradie1,2,* Brian Reeves3, Sandile Mdoko3, Lwandiso Pamla3, and Oyama Gxabhu3 Abstract. The results of a herpetofaunal survey of Ongeluksnek Nature Reserve, Eastern Cape Province, South Africa are presented here. Combination of visual encounter survey methods and standard Y-shape trap arrays were used to conduct the survey. A total of 26 species (eight amphibians and 18 reptiles) were recorded, representing 29 quarter-degree grid cell records, of which 62% represented the first records for these units. Furthermore, we document the presence of three species of snakes (Crotaphopeltis hotamboeia, Hemachatus haemachatus and Homoroselaps lacteus) for the first time for the whole degree square of 3028 (approx. 100 km2). This study highlights the need to survey poorly known regions to enable us to understand and document the full distributional extent of species. We also discuss the impact of uncontrolled fires on the absence of grassland specialised species during our survey. Keywords. Amphibia, Reptilia, karroid, conservation, biodiversity, fire Introduction has been done in the southern and western regions (e.g. Branch and Braack, 1987), while the northern and The herpetofaunal richness of South Africa is central areas associated with the former homelands of considered to be amongst the highest in the world the Ciskei and Transkei remained poorly surveyed. In (Branch, 1998; Bates et al., 2014; Du Preez and recent years a series of rapid biodiversity studies has Carruthers, 2017; Tolley et al., 2019). -

Epigaeic Invertebrate Community Structure in Two Subtropical Nature Reserves, Eastern Cape, South Africa: Implications for Conservation Management
© Arachnologische Gesellschaft e.V. Frankfurt/Main; http://arages.de/ Arachnologische Mitteilungen / Arachnology Letters 52: 7-15 Karlsruhe, September 2016 Epigaeic invertebrate community structure in two subtropical nature reserves, Eastern Cape, South Africa: Implications for conservation management Augustine S. Niba & Inam Yekwayo doi: 10.5431/aramit5203 Abstract. Epigaeic invertebrates were sampled at non-invaded (‘Indigenous Forest’ and ‘Indigenous Grassland’) and alien-invaded (‘Eu- calyptus’ and ‘Mixed alien’) sub-sites in the Nduli and Luchaba Nature Reserves using pitfall traps. A total of 2054 specimens belonging to three phyla (Arthropoda, Mollusca and Annelida) was caught and sorted into seven orders, 18 families, one tribe, 45 genera (22 identified to species level) and 20 morphospecies. Higher species richness occurred in ‘Indigenous Forest’ and ‘Mixed Alien’ sub-sites while higher specimen counts were made in invaded (‘Mixed Alien’ and ‘Eucalyptus’) sub-sites during summer months, peaking in January. Canonical Correspondence Analysis results show that some measured site variables, e.g. litter depth, grazing intensity, percentage of alien vege- tation cover, and soil chemical properties accounted for invertebrate taxa composition and distribution trends at sub-sites. Although habitat-patch level characteristics (including abiotic factors) were important for determining species distributions, increased levels of infestation by invasive alien vegetation across sub-sites did not necessarily impact on epigaeic invertebrates -

Climate Forcing of Tree Growth in Dry Afromontane Forest Fragments of Northern Ethiopia: Evidence from Multi-Species Responses Zenebe Girmay Siyum1,2* , J
Siyum et al. Forest Ecosystems (2019) 6:15 https://doi.org/10.1186/s40663-019-0178-y RESEARCH Open Access Climate forcing of tree growth in dry Afromontane forest fragments of Northern Ethiopia: evidence from multi-species responses Zenebe Girmay Siyum1,2* , J. O. Ayoade3, M. A. Onilude4 and Motuma Tolera Feyissa2 Abstract Background: Climate-induced challenge remains a growing concern in the dry tropics, threatening carbon sink potential of tropical dry forests. Hence, understanding their responses to the changing climate is of high priority to facilitate sustainable management of the remnant dry forests. In this study, we examined the long-term climate- growth relations of main tree species in the remnant dry Afromontane forests in northern Ethiopia. The aim of this study was to assess the dendrochronological potential of selected dry Afromontane tree species and to study the influence of climatic variables (temperature and rainfall) on radial growth. It was hypothesized that there are potential tree species with discernible annual growth rings owing to the uni-modality of rainfall in the region. Ring width measurements were based on increment core samples and stem discs collected from a total of 106 trees belonging to three tree species (Juniperus procera, Olea europaea subsp. cuspidate and Podocarpus falcatus). The collected samples were prepared, crossdated, and analyzed using standard dendrochronological methods. The formation of annual growth rings of the study species was verified based on successful crossdatability and by correlating tree-ring widths with rainfall. Results: The results showed that all the sampled tree species form distinct growth boundaries though differences in the distinctiveness were observed among the species. -

Lesotho Fourth National Report on Implementation of Convention on Biological Diversity
Lesotho Fourth National Report On Implementation of Convention on Biological Diversity December 2009 LIST OF ABBREVIATIONS AND ACRONYMS ADB African Development Bank CBD Convention on Biological Diversity CCF Community Conservation Forum CITES Convention on International Trade in Endangered Species CMBSL Conserving Mountain Biodiversity in Southern Lesotho COP Conference of Parties CPA Cattle Post Areas DANCED Danish Cooperation for Environment and Development DDT Di-nitro Di-phenyl Trichloroethane EA Environmental Assessment EIA Environmental Impact Assessment EMP Environmental Management Plan ERMA Environmental Resources Management Area EMPR Environmental Management for Poverty Reduction EPAP Environmental Policy and Action Plan EU Environmental Unit (s) GA Grazing Associations GCM Global Circulation Model GEF Global Environment Facility GMO Genetically Modified Organism (s) HIV/AIDS Human Immuno Virus/Acquired Immuno-Deficiency Syndrome HNRRIEP Highlands Natural Resources and Rural Income Enhancement Project IGP Income Generation Project (s) IUCN International Union for Conservation of Nature and Natural Resources LHDA Lesotho Highlands Development Authority LMO Living Modified Organism (s) Masl Meters above sea level MDTP Maloti-Drakensberg Transfrontier Conservation and Development Project MEAs Multi-lateral Environmental Agreements MOU Memorandum Of Understanding MRA Managed Resource Area NAP National Action Plan NBF National Biosafety Framework NBSAP National Biodiversity Strategy and Action Plan NEAP National Environmental Action -

Comparative Wood Anatomy of Afromontane and Bushveld Species from Swaziland, Southern Africa
IAWA Bulletin n.s., Vol. 11 (4), 1990: 319-336 COMPARATIVE WOOD ANATOMY OF AFROMONTANE AND BUSHVELD SPECIES FROM SWAZILAND, SOUTHERN AFRICA by J. A. B. Prior 1 and P. E. Gasson 2 1 Department of Biology, Imperial College of Science, Technology & Medicine, London SW7 2BB, U.K. and 2Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3DS, U.K. Summary The habit, specific gravity and wood anat of the archaeological research, uses all the omy of 43 Afromontane and 50 Bushveld well preserved, qualitative anatomical charac species from Swaziland are compared, using ters apparent in the charred modem samples qualitative features from SEM photographs in an anatomical comparison between the of charred samples. Woods with solitary ves two selected assemblages of trees and shrubs sels, scalariform perforation plates and fibres growing in areas of contrasting floristic com with distinctly bordered pits are more com position. Some of the woods are described in mon in the Afromontane species, whereas Kromhout (1975), others are of little com homocellular rays and prismatic crystals of mercial importance and have not previously calcium oxalate are more common in woods been investigated. Few ecological trends in from the Bushveld. wood anatomical features have previously Key words: Swaziland, Afromontane, Bush been published for southern Africa. veld, archaeological charcoal, SEM, eco The site of Sibebe Hill in northwest Swazi logical anatomy. land (26° 15' S, 31° 10' E) (Price Williams 1981), lies at an altitude of 1400 m, amidst a Introduction dramatic series of granite domes in the Afro Swaziland, one of the smallest African montane forest belt (White 1978). -

Annual Report Annual
EASTERN CAPE PARKS AND TOURISM ANNUAL REPORT 2018/19 REPORT ANNUAL AND TOURISM EASTERN CAPE PARKS 17-25 Oxford Street | East London | 5213 East London | PO Box 11235 | Southernwood | East London | 5213 Eastern Cape | South Africa +27 (0) 43 492 0881 [email protected] www.visiteasterncape.co.za RP290/2019 ISBN: 978-0-621-47767-2 ANNUAL REPORT Long-beaked common dolphin COVER Photo credit: Mark M Gottlieb – www.MarksAdventures.com Hole in the Wall Photo credit: Marius Labuschagne Pounding waves have helped shape South Africa’s coastline for 160 million years - since continental rifting tore Africa from the ancestral landmass Gondwana, leaving her edges exposed to a unique blend of currents and seas. National Geographic Gondwana was an ancient supercontinent that broke up about 180 million years ago. The continent eventually split into landmasses we recognize today: Africa, South America, Australia, Antarctica, the Indian subcontinent and the Arabian Peninsula. CONTENTS PG 5 GENERAL INFORMATION 1 PUBLIC.................................................................................................. ENTITY’S GENERAL INFORMATION 7 2 BO..................................................................................................ARD MEMBERS 8 3 LI...............................................................................................ST OF ABBREVIATIONS/ACRONYMS 10 1 AUDITOR’S REPORT: PREDETERMINED OBJECTIVES 4 FO................................................................................................REWORD BY THE CHAIRPERSON -
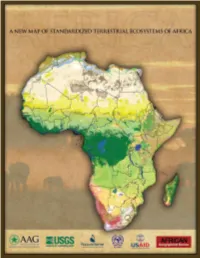
A New Map of Standardized Terrestrial Ecosystems of Africa
Major contributors to this publication include: The Association of American Geographers is a nonprofit scientific and educational society with a membership of over 10,500 individuals from more than 60 countries. AAG members are geographers and related professionals who work in the public, private, and academic sectors to advance the theory, methods, and practice of geography. This booklet is published by AAG as a special supplement to the African Geographical Review. The U.S. Geological Survey (USGS) was created in 1879 as a science agency charged with providing information and understanding to help resolve complex natural resource problems across the nation and around the world. The mission of the USGS is to provide relevant, impartial scientific information to 1) describe and understand the Earth, 2) minimize loss of life and property from natural disasters, 3) manage water, biological, energy, and mineral resources, and 4) enhance and protect our quality of life. NatureServe is an international conservation nonprofit dedicated to providing the sci- entific basis for effective conservation action. NatureServe’s network in the Americas includes more than 80 member institutions that collect and maintain a unique body of scientific knowledge about the species and ecosystems. The information products, data management tools, and biodiversity expertise that NatureServe’s scientists, technologists, and other professionals provide help meet local, national, and global conservation needs. The Regional Centre for Mapping of Resources for Development (RCMRD) was es- tablished in Nairobi, Kenya in 1975 to provide quality Geo-Information and allied Information Technology products and services in environmental and resource manage- ment for sustainable development in our member countries and beyond. -

Fire Regimes in Eastern Coastal Fynbos
Fire regimes in eastern coastal fynbos: drivers, ecology and management by Tineke Kraaij Submitted in fulfilment/partial fulfilment of the requirements for the degree of Doctorate in Philosophy in the Faculty of Science at the Nelson Mandela Metropolitan University August 2012 Promotor: Prof. R.M. Cowling Co-promotor: Dr B.W. van Wilgen Declaration I, Tineke Kraaij, student number 211211583, hereby declare that the thesis for Doctorate of Philosophy is my own work and that it has not previously been submitted for assessment or completion of any postgraduate qualification to another University or for another qualification. I am now presenting the thesis for examination for the degree of Doctorate of Philosophy. Tineke Kraaij Table of Contents Abstract ..................................................................................................................................... 5 Acknowledgements .................................................................................................................... 7 List of Tables .............................................................................................................................. 9 List of Figures ........................................................................................................................... 10 Introduction ............................................................................................................................. 11 References .................................................................................................................................................. -

Cape Parrot Big Birding Day Report
17th Annual Parrot Count- Report on the 2014 Cape Parrot Big Birding Day. Colleen T. Downs* School of Life Sciences, University of KwaZulu-Natal, P/Bag X01, Scottsville, 3209, South Africa. Email: [email protected] *Cape Parrot Working Group Chairperson. Background The Cape Parrot (Poicephalus robustus) is the only parrot species endemic to South Africa and is currently one of South Africa’s Endangered birds. Skead (1971) highlighted that the numbers of Cape Parrots had declined since the early 1900s, particularly in the 1950’s. In 1989 Boshoff estimated less than a 1000 in the wild. So this raised questions: were these declines real and how many Cape parrots were there in the wild? Standard bird counting techniques are unsuitable for Cape Parrots as they are nomadic feeders with unpredictable movements. Parrots’ cryptic colouration combined with dense forest habitats often make them difficult to locate once perched but their loud harsh calls whilst in-flight make their presence known. They are most active during the first few hours after dawn and before sunset, (although during misty conditions these periods can be extended), when they leave and return to their roosts in forest patches. These characteristics allow for a ‘total count’ of the parrots. Consequently the Cape Parrot Big Birding Day (CPBBD) was initiated in 1998, and has been held annually since as part of the conservation effort of the Cape Parrot Working Group. The aim is to determine their occurrence and obtain an accurate population estimate of the Cape Parrot. Over the recent years less than 1600 have been counted in the wild (Downs et al. -

Eastern Cape Biodiversity Conservation Plan Technical Report
EASTERN CAPE BIODIVERSITY CONSERVATION PLAN TECHNICAL REPORT Derek Berliner & Philip Desmet “Mainstreaming Biodiversity in Land Use Decision- Making in the Eastern Cape Province” DWAF Project No 2005-012 1 August 2007 Revision 1 (5 September 2005) Eastern Cape Biodiversity Conservation Plan Technical Report I Photo by Barry Clark Report Title; Eastern Cape Biodiversity Conservation Plan Technical Report. Date: 1 August 2007 Authors: Derek Berliner & Dr Phillip Desmet Contact details; Derek Berliner, Eco-logic Consulting, email: [email protected]. cell: 083 236 7155 Dr Phillip Desmet, email: [email protected], cell: 082 352 2955 Client: Department of Water Affairs and Forestry Principle funding agent: Development Bank of South Africa Citation: Berliner D. & Desmet P. (2007) Eastern Cape Biodiversity Conservation Plan: Technical Report. Department of Water Affairs and Forestry Project No 2005-012, Pretoria. 1 August 2007 (Unless otherwise quoted, intellectual property rights for the conceptual content of this report reside with the above authors) Eastern Cape Biodiversity Conservation Plan Technical Report II Acknowledgements The assistance of a large number of people has been essential to the success of this project. In particular, the authors would like to thank the funders of this project, the DBSA and DWAF, Nkosi Quvile (DWAF), Phumla Mzazi (DEDEA), Mandy Driver (SANBI), Julie Clarke (DBSA), Graeme Harrison (formerly DWAF) and members of the Project Steering Committee and Eastern Cape Implementation Committee for Bioregional Programmes. Our thanks also go to Ally Ashwell, John Allwood, Dave Balfour, Noluthando Bam, Rick Bernard, Roger Bills, Anton Bok, Andre Boshoff, Bill Branch, Mandy Cadman, Jim Cambray, Barry Clark, Willem Coetzer, P. -

The Maloti Drakensberg Experience See Travel Map Inside This Flap❯❯❯
exploring the maloti drakensberg route the maloti drakensberg experience see travel map inside this flap❯❯❯ the maloti drakensberg experience the maloti drakensberg experience …the person who practices ecotourism has the opportunity of immersing him or herself in nature in a way that most people cannot enjoy in their routine, urban existences. This person “will eventually acquire a consciousness and knowledge of the natural environment, together with its cultural aspects, that will convert him or her into somebody keenly involved in conservation issues… héctor ceballos-lascuráin internationally renowned ecotourism expert” travel tips for the maloti drakensberg region Eastern Cape Tourism Board +27 (0)43 701 9600 www.ectb.co.za, [email protected] lesotho south africa Ezemvelo KZN Wildlife currency Maloti (M), divided into 100 lisente (cents), have currency The Rand (R) is divided into 100 cents. Most +27 (0)33 845 1999 an equivalent value to South African rand which are used traveller’s cheques are accepted at banks and at some shops www.kznwildlife.com; [email protected] interchangeably in Lesotho. Note that Maloti are not accepted and hotels. Major credit cards are accepted in most towns. Free State Tourism Authority in South Africa in place of rand. banks All towns will have at least one bank. Open Mon to Fri: +27 (0)51 411 4300 Traveller’s cheques and major credit cards are generally 09h00–15h30, Sat: 09h00–11h00. Autobanks (or ATMs) are www.dteea.fs.gov.za accepted in Maseru. All foreign currency exchange should be found in most towns and operate on a 24-hour basis.