Effects of Atmospheric CO2 Variability of the Past 800 Kyr on the Biomes of Southeast Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biodiversity and Ecology of Afromontane Rainforests with Wild Coffea Arabica L
Feyera Senbeta Wakjira (Autor) Biodiversity and ecology of Afromontane rainforests with wild Coffea arabica L. populations in Ethiopia https://cuvillier.de/de/shop/publications/2260 Copyright: Cuvillier Verlag, Inhaberin Annette Jentzsch-Cuvillier, Nonnenstieg 8, 37075 Göttingen, Germany Telefon: +49 (0)551 54724-0, E-Mail: [email protected], Website: https://cuvillier.de General introduction 1 GENERAL INTRODUCTION 1.1 Background The term biodiversity is used to convey the total number, variety and variability of living organisms and the ecological complexes in which they occur (Wilson 1988; CBD 1992; Rosenzweig 1995). The concept of biological diversity can be applied to a wide range of spatial and organization scales, including genetics, species, community, and landscape scales (Noss 1990; Austin et al. 1996; Tuomisto et al. 2003). It is becoming increasingly apparent that knowledge of the role of patterns and processes that determine diversity at different scales is at the very heart of an understanding of variation in biodiversity. Processes influencing diversity operate at different spatial and temporal scales (Rosenzweig 1995; Gaston 2000). A variety of environmental events and processes, including past evolutionary development, biogeographic processes, extinctions, and current influences govern the biodiversity of a particular site (Brown and Lomolino 1998; Gaston 2000; Ricklefs and Miller 2000). Biodiversity is valued and has been studied largely because it is used, and could be used better, to sustain and improve human well-being (WWF 1993; WCMC 1994). However, there has been a rapid decline in the biodiversity of the world during the past two to three decades (Wilson 1988; Whitmore and Sayer 1992; Lugo et al. -

Martian Crater Morphology
ANALYSIS OF THE DEPTH-DIAMETER RELATIONSHIP OF MARTIAN CRATERS A Capstone Experience Thesis Presented by Jared Howenstine Completion Date: May 2006 Approved By: Professor M. Darby Dyar, Astronomy Professor Christopher Condit, Geology Professor Judith Young, Astronomy Abstract Title: Analysis of the Depth-Diameter Relationship of Martian Craters Author: Jared Howenstine, Astronomy Approved By: Judith Young, Astronomy Approved By: M. Darby Dyar, Astronomy Approved By: Christopher Condit, Geology CE Type: Departmental Honors Project Using a gridded version of maritan topography with the computer program Gridview, this project studied the depth-diameter relationship of martian impact craters. The work encompasses 361 profiles of impacts with diameters larger than 15 kilometers and is a continuation of work that was started at the Lunar and Planetary Institute in Houston, Texas under the guidance of Dr. Walter S. Keifer. Using the most ‘pristine,’ or deepest craters in the data a depth-diameter relationship was determined: d = 0.610D 0.327 , where d is the depth of the crater and D is the diameter of the crater, both in kilometers. This relationship can then be used to estimate the theoretical depth of any impact radius, and therefore can be used to estimate the pristine shape of the crater. With a depth-diameter ratio for a particular crater, the measured depth can then be compared to this theoretical value and an estimate of the amount of material within the crater, or fill, can then be calculated. The data includes 140 named impact craters, 3 basins, and 218 other impacts. The named data encompasses all named impact structures of greater than 100 kilometers in diameter. -

Ignimbrites to Batholiths Ignimbrites to Batholiths: Integrating Perspectives from Geological, Geophysical, and Geochronological Data
Ignimbrites to batholiths Ignimbrites to batholiths: Integrating perspectives from geological, geophysical, and geochronological data Peter W. Lipman1,* and Olivier Bachmann2 1U.S. Geological Survey, Mail Stop 910, Menlo Park, California 94028, USA 2Institute of Geochemistry and Petrology, ETH Zurich, CH-8092 Zürich, Switzerland ABSTRACT related intrusions cooled and solidified soon shorter. Magma-supply estimates (from ages after zircon crystallization, as magma sup- and volcano-plutonic volumes) yield focused Multistage histories of incremental accu- ply waned. Some researchers interpret these intrusion-assembly rates sufficient to gener- mulation, fractionation, and solidification results as recording pluton assembly in small ate ignimbrite-scale volumes of eruptible during construction of large subvolcanic increments that crystallized rapidly, leading magma, based on published thermal models. magma bodies that remained sufficiently to temporal disconnects between ignimbrite Mid-Tertiary processes of batholith assembly liquid to erupt are recorded by Tertiary eruption and intrusion growth. Alternatively, associated with the SRMVF caused drastic ignimbrites, source calderas, and granitoid crystallization ages of the granitic rocks chemical and physical reconstruction of the intrusions associated with large gravity lows are here inferred to record late solidifica- entire lithosphere, probably accompanied by at the Southern Rocky Mountain volcanic tion, after protracted open-system evolution asthenospheric input. field (SRMVF). Geophysical -

Climate Forcing of Tree Growth in Dry Afromontane Forest Fragments of Northern Ethiopia: Evidence from Multi-Species Responses Zenebe Girmay Siyum1,2* , J
Siyum et al. Forest Ecosystems (2019) 6:15 https://doi.org/10.1186/s40663-019-0178-y RESEARCH Open Access Climate forcing of tree growth in dry Afromontane forest fragments of Northern Ethiopia: evidence from multi-species responses Zenebe Girmay Siyum1,2* , J. O. Ayoade3, M. A. Onilude4 and Motuma Tolera Feyissa2 Abstract Background: Climate-induced challenge remains a growing concern in the dry tropics, threatening carbon sink potential of tropical dry forests. Hence, understanding their responses to the changing climate is of high priority to facilitate sustainable management of the remnant dry forests. In this study, we examined the long-term climate- growth relations of main tree species in the remnant dry Afromontane forests in northern Ethiopia. The aim of this study was to assess the dendrochronological potential of selected dry Afromontane tree species and to study the influence of climatic variables (temperature and rainfall) on radial growth. It was hypothesized that there are potential tree species with discernible annual growth rings owing to the uni-modality of rainfall in the region. Ring width measurements were based on increment core samples and stem discs collected from a total of 106 trees belonging to three tree species (Juniperus procera, Olea europaea subsp. cuspidate and Podocarpus falcatus). The collected samples were prepared, crossdated, and analyzed using standard dendrochronological methods. The formation of annual growth rings of the study species was verified based on successful crossdatability and by correlating tree-ring widths with rainfall. Results: The results showed that all the sampled tree species form distinct growth boundaries though differences in the distinctiveness were observed among the species. -

Warfare in a Fragile World: Military Impact on the Human Environment
Recent Slprt•• books World Armaments and Disarmament: SIPRI Yearbook 1979 World Armaments and Disarmament: SIPRI Yearbooks 1968-1979, Cumulative Index Nuclear Energy and Nuclear Weapon Proliferation Other related •• 8lprt books Ecological Consequences of the Second Ihdochina War Weapons of Mass Destruction and the Environment Publish~d on behalf of SIPRI by Taylor & Francis Ltd 10-14 Macklin Street London WC2B 5NF Distributed in the USA by Crane, Russak & Company Inc 3 East 44th Street New York NY 10017 USA and in Scandinavia by Almqvist & WikseH International PO Box 62 S-101 20 Stockholm Sweden For a complete list of SIPRI publications write to SIPRI Sveavagen 166 , S-113 46 Stockholm Sweden Stoekholol International Peace Research Institute Warfare in a Fragile World Military Impact onthe Human Environment Stockholm International Peace Research Institute SIPRI is an independent institute for research into problems of peace and conflict, especially those of disarmament and arms regulation. It was established in 1966 to commemorate Sweden's 150 years of unbroken peace. The Institute is financed by the Swedish Parliament. The staff, the Governing Board and the Scientific Council are international. As a consultative body, the Scientific Council is not responsible for the views expressed in the publications of the Institute. Governing Board Dr Rolf Bjornerstedt, Chairman (Sweden) Professor Robert Neild, Vice-Chairman (United Kingdom) Mr Tim Greve (Norway) Academician Ivan M£ilek (Czechoslovakia) Professor Leo Mates (Yugoslavia) Professor -

Lesotho Fourth National Report on Implementation of Convention on Biological Diversity
Lesotho Fourth National Report On Implementation of Convention on Biological Diversity December 2009 LIST OF ABBREVIATIONS AND ACRONYMS ADB African Development Bank CBD Convention on Biological Diversity CCF Community Conservation Forum CITES Convention on International Trade in Endangered Species CMBSL Conserving Mountain Biodiversity in Southern Lesotho COP Conference of Parties CPA Cattle Post Areas DANCED Danish Cooperation for Environment and Development DDT Di-nitro Di-phenyl Trichloroethane EA Environmental Assessment EIA Environmental Impact Assessment EMP Environmental Management Plan ERMA Environmental Resources Management Area EMPR Environmental Management for Poverty Reduction EPAP Environmental Policy and Action Plan EU Environmental Unit (s) GA Grazing Associations GCM Global Circulation Model GEF Global Environment Facility GMO Genetically Modified Organism (s) HIV/AIDS Human Immuno Virus/Acquired Immuno-Deficiency Syndrome HNRRIEP Highlands Natural Resources and Rural Income Enhancement Project IGP Income Generation Project (s) IUCN International Union for Conservation of Nature and Natural Resources LHDA Lesotho Highlands Development Authority LMO Living Modified Organism (s) Masl Meters above sea level MDTP Maloti-Drakensberg Transfrontier Conservation and Development Project MEAs Multi-lateral Environmental Agreements MOU Memorandum Of Understanding MRA Managed Resource Area NAP National Action Plan NBF National Biosafety Framework NBSAP National Biodiversity Strategy and Action Plan NEAP National Environmental Action -

Comparative Wood Anatomy of Afromontane and Bushveld Species from Swaziland, Southern Africa
IAWA Bulletin n.s., Vol. 11 (4), 1990: 319-336 COMPARATIVE WOOD ANATOMY OF AFROMONTANE AND BUSHVELD SPECIES FROM SWAZILAND, SOUTHERN AFRICA by J. A. B. Prior 1 and P. E. Gasson 2 1 Department of Biology, Imperial College of Science, Technology & Medicine, London SW7 2BB, U.K. and 2Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3DS, U.K. Summary The habit, specific gravity and wood anat of the archaeological research, uses all the omy of 43 Afromontane and 50 Bushveld well preserved, qualitative anatomical charac species from Swaziland are compared, using ters apparent in the charred modem samples qualitative features from SEM photographs in an anatomical comparison between the of charred samples. Woods with solitary ves two selected assemblages of trees and shrubs sels, scalariform perforation plates and fibres growing in areas of contrasting floristic com with distinctly bordered pits are more com position. Some of the woods are described in mon in the Afromontane species, whereas Kromhout (1975), others are of little com homocellular rays and prismatic crystals of mercial importance and have not previously calcium oxalate are more common in woods been investigated. Few ecological trends in from the Bushveld. wood anatomical features have previously Key words: Swaziland, Afromontane, Bush been published for southern Africa. veld, archaeological charcoal, SEM, eco The site of Sibebe Hill in northwest Swazi logical anatomy. land (26° 15' S, 31° 10' E) (Price Williams 1981), lies at an altitude of 1400 m, amidst a Introduction dramatic series of granite domes in the Afro Swaziland, one of the smallest African montane forest belt (White 1978). -
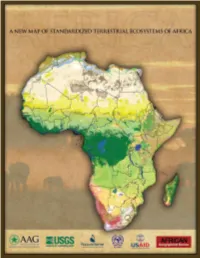
A New Map of Standardized Terrestrial Ecosystems of Africa
Major contributors to this publication include: The Association of American Geographers is a nonprofit scientific and educational society with a membership of over 10,500 individuals from more than 60 countries. AAG members are geographers and related professionals who work in the public, private, and academic sectors to advance the theory, methods, and practice of geography. This booklet is published by AAG as a special supplement to the African Geographical Review. The U.S. Geological Survey (USGS) was created in 1879 as a science agency charged with providing information and understanding to help resolve complex natural resource problems across the nation and around the world. The mission of the USGS is to provide relevant, impartial scientific information to 1) describe and understand the Earth, 2) minimize loss of life and property from natural disasters, 3) manage water, biological, energy, and mineral resources, and 4) enhance and protect our quality of life. NatureServe is an international conservation nonprofit dedicated to providing the sci- entific basis for effective conservation action. NatureServe’s network in the Americas includes more than 80 member institutions that collect and maintain a unique body of scientific knowledge about the species and ecosystems. The information products, data management tools, and biodiversity expertise that NatureServe’s scientists, technologists, and other professionals provide help meet local, national, and global conservation needs. The Regional Centre for Mapping of Resources for Development (RCMRD) was es- tablished in Nairobi, Kenya in 1975 to provide quality Geo-Information and allied Information Technology products and services in environmental and resource manage- ment for sustainable development in our member countries and beyond. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Corrosion Map of South Africa's Macro Atmosphere
Corrosion map of South Africa’s macro atmosphere AUTHORS: Darelle T. Janse van Rensburg1,2 The first atmospheric corrosion map of South Africa, produced by Callaghan in 1991, has become outdated, Lesley A. Cornish1 because it primarily focuses on the corrosivity of coastal environments, with little differentiation given Josias van der Merwe1 concerning South Africa’s inland locations. To address this problem, a study was undertaken to develop AFFILIATIONS: a new corrosion map of the country, with the emphasis placed on providing greater detail concerning 1School of Chemical and Metallurgical South Africa’s inland regions. Here we present this new corrosion map of South Africa’s macro atmosphere, Engineering and DST-NRF Centre of Excellence in Strong Materials, based on 12-month corrosion rates of mild steel at more than 100 sites throughout the country. Assimilations University of the Witwatersrand, and statistical analyses of the data (published, unpublished and new) show that the variability in the corrosion Johannesburg, South Africa rate of mild steel decreases significantly moving inland. Accordingly, the average first-year corrosion rate of 2Orytech (Pty) Ltd, Roodepoort, South Africa mild steel at the inland sites (at all corrosion monitoring spots located more than 30 km away from the ocean) measured 21±12 µm/a [95% CI: 18–23 µm/a]. The minimum inland figure was about 1.3 µm/a (recorded CORRESPONDENCE TO: at Droërivier in the Central Karoo) and the maxima were approximately 51 µm/a and 50 µm/a in the industrial Darelle Janse van Rensburg hearts of Germiston (Gauteng) and Sasolburg (Free State), respectively. -

Distribution, Habitat Structure and Troop Size in Eastern Cape
Distribution, habitat structure and troop size in Eastern Cape samango monkeys Cercopithecus albogularis labiatus (Primates: Cercopithecoidea) A dissertation submitted to the Department of Zoology and Entomology in fulfilment of the requirements for the degree of Master of Science in Zoology the University of Fort Hare by Vusumzi Martins Supervisors: Dr Fabien Génin and Prof. Judith Masters GENERAL ABSTRACT The samango monkey subspecies Cercopithecus albogularis labiatus is endemic to South Africa, and known to occur in Afromontane forests. There has been a major decline in this subspecies, exceeding 30% in some populations over the past 30 years, primarily as a result of the loss of suitable habitat. A second samango subspecies, C. a. erythrarchus, occurs near the northern border of South Africa, mainly in coastal lowland forest, and the distributions of the two subspecies do not overlap. C. a. labiatus was thought to be confined to Afromontane forests, but the study described here focused on C. a. labiatus populations that were recently identified in the Indian Ocean Belt forests near East London. I undertook to assess the distribution of C. a. labiatus in the Eastern Cape, to evaluate the habitat structures of the Afromontane and Indian Ocean coastal belt forests, and to understand the effect these habitats have on essential aspects of the socio-ecology of the C. a. labiatus populations. Distribution surveys were conducted in protected areas throughout the Eastern Cape, and samango monkeys were found to be present within forest patches in the Amatola Mountains, Eastern Cape dune forests and the Transkei coastal scarp forests. The sizes and composition of two troops were assessed: one troop in the Amatole forests and one in the Eastern Cape dune forests. -

Crater Ice Deposits Near the South Pole of Mars Owen William Westbrook
Crater Ice Deposits Near the South Pole of Mars by Owen William Westbrook Submitted to the Department of Earth, Atmospheric, and Planetary Sciences in partial fulfillment of the requirements for the degree of Master of Science in Earth and Planetary Sciences at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2009 © Massachusetts Institute of Technology 2009. All rights reserved. A uth or ........................................ Department of Earth, Atmospheric, and Planetary Sciences May 22, 2009 Certified by . Maria T. Zuber E. A. Griswold Professor of Geophysics Thesis Supervisor 6- Accepted by.... ...... ..... ........................................... Daniel Rothman Professor of Geophysics Department of Earth, Atmospheric and Planetary Sciences MASSACHUSETTS INSTWITE OF TECHNOLOGY JUL 2 0 2009 ARCHIES LIBRARIES Crater Ice Deposits Near the South Pole of Mars by Owen William Westbrook Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on May 22, 2009, in partial fulfillment of the requirements for the degree of Master of Science in Earth and Planetary Sciences Abstract Layered deposits atop both Martian poles are thought to preserve a record of past climatic conditions in up to three km of water ice and dust. Just beyond the extent of these south polar layered deposits (SPLD), dozens of impact craters contain large mounds of fill material with distinct similarities to the main layered deposits. Previously identified as outliers of the main SPLD, these deposits could offer clues to the climatic history of the Martian south polar region. We extend previous studies of these features by cataloging all crater deposits found near the south pole and quantifying the physical parameters of both the deposits and their host craters.