Influence of Grape-Associated Filamentous Fungi and Their Exoproteome on the Gushing in Sparkling Wine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wine Proteins: Origin, Characteristics and Functionality Andrea Curioni
The wine proteins: origin, characteristics and functionality Andrea Curioni Dipartimento di Biotecnologie Agrarie Centro interdipartimentale per la Ricerca in Viticoltura ed Enologia (CIRVE) Università di Padova 1 The CIRVE campus in Conegliano 2 Protein Structure / Functionality Aminoacid sequence Protein Protein structure • Size • Charge • Hydrophobicity Proprieties Functionality Environment Detectable • pH • Solvent effects • Ionic strength • Temperature • Etc. 3 Proteins in wine Implications in wine –Hazing of white wines (negative) –“Mouthfeel” and aroma –Foam volume and stability The wine proteins Tarragona 2011 4 Protein Haze in wine Serious quality defect Prevention: Protein removal by bentonite treatments Bottled wine Flocculation Coagulation Precipitation Bentonite Other methods? several drawbacks: • Loss of aroma Knowledge is • Cost needed • Waste • ….. The wine proteins Tarragona 2011 5 Wine Proteins: Origin Where do the wine proteins came from? The wine proteins Tarragona 2011 6 Wine Proteins: Origin • The wine proteins derive from Grape (mainly): involved in wine hazing Microorganisms The wine proteins Tarragona 2011 7 Grape Proteins • Accumulate after veraison – with sugars • Low quantity – ≈ hundreds mg/Kg • heterogeneous - > 300 components • Few main components Pocock et al. (2000) JAFC 48, 1637 The wine proteins Tarragona 2011 8 The Grape Proteins similar in all the varieties Sarry et al., 2004 Proteomics, 4, 201 pH The wine proteins Tarragona 2011 9 Grape Proteins: Identification by MS PR-proteins Sarry et al., 2004 Proteomics, 4, 201 10 The wine proteins Tarragona 2011 Grape Proteins: the main components Pathogenesis related (PR)-Proteins – THAUMATIN-LIKE PROTEINS (TLP, PR 5) • ≈ 24 kDa – CHITINASES (PR 3) • ≈ 30 kDa – Osmotins – Beta-(1,3)-glucanases The wine proteins Tarragona 201111 Thaumatin-like Proteins (TLP) • Antifungal activity • Expressed mainly in the berry • Several types – main: VvTL1 (constitutive) – minor : VvTL2 (less present in healthy grapes), . -

The Role and Use of Non-Saccharomyces Yeasts in Wine Production
The Role and Use of Non-Saccharomyces Yeasts in Wine Production N.P. Jolly1*, O.P.H. Augustyn1 and I.S. Pretorius2** (1) ARC Infruitec-Nietvoorbij***, Private Bag X5026, 7599 Stellenbosch, South Africa. (2) Institute for Wine Biotechnology, Department of Viticulture & Oenology, Stellenbosch University, Private Bag X1, 7602 Matieland (Stellenbosch), South Africa. Submitted for publication: September 2005 Accepted for publication: April 2006 Key words: Non-Saccharomyces, yeasts, vineyards, cellars, fermentation, wine. The contribution by the numerous grape-must-associated non-Saccharomyces yeasts to wine fermentation has been debated extensively. These yeasts, naturally present in all wine fermentations, are metabolically active and their metabolites can impact on wine quality. Although often seen as a source of microbial spoilage, there is substantial contrary evidence pointing to a positive contribution by these yeasts. The role of non-Saccharomyces yeasts in wine fermentation is therefore receiving increasing attention by wine microbiologists in Old and New World wine producing countries. Species that have been investigated for wine production thus far include those from the Candida, Kloeckera, Hanseniaspora, Zygosaccharomyces, Schizosaccharomyces, Torulaspora, Brettanomyces, Saccharomycodes, Pichia and Williopsis genera. In this review the use and role of non-Saccharomyces yeast in wine production is presented and research trends are discussed. INTRODUCTION roles of the numerous non-Saccharomyces yeasts normally asso- ciated with grape must and wine. These yeasts, naturally present Wine is the product of a complex biological and biochemical in all wine fermentations to a greater or lesser extent, are meta- interaction between grapes (grape juice) and different microor- bolically active and their metabolites can impact on wine quality. -

The Path to Bottling
TANKS The Path to Bottling www.laffort.com YOUR ACCESS TO 120 YEARS OF WINEMAKING INNOVATION Save it in your favourites now for 24/7 access to ! PRODUCT INFORMATION SHEETS QUALITY DOCUMENTS ( HACCP & ISO ) PRODUCT SAFETY SHEETS RESEARCH PAPERS DECISION MAKING TOOLS CATALOGUE DOWNLOADS PERSONALISED NUTRITION PUBLISHED ARTICLES CALCULATOR CERTIFICATES OF ANALYSIS ORGANIC CERTIFICATIONS TRAINING VIDEOS LAFFORT® PLANT BASED INNOVATIONS LAFFORT® unrivalled technical resources is delivering the most scientifically advanced oenological solutions from plant derived products. VEGAN FRIENDLY & ORGANIC THEY’RE NOT JUST OPTIONS THEY’RE SUPERIOR SOLUTIONS These symbols are a guide to your LAFFORT® products properties. GANI TAL OR LERGE OR C E IG L N G I A E N V A L E L E S E R UIT ABLE RGEN F F R E E Organic certification bodies have different criteria for certification and products may differ from one certification body to another. Please contact your certifying agent to confirm a products organic certification. PROTECTING YOUR WHITE WINE MANAGEMENT WHITE WINE PROTECTING YOUR WHITE WINE - A TRADITIONAL APPROACH White wines are vulnerable to oxidation and microbial changes post alcoholic fermentation. Microbial and anti-oxidative control of white wines is a first step to getting wines ready for bottling and/or storage. Threats of oxygen on finished white wines; • Proliferation of acetic acid bacteria. • Proliferation of Brettanomyces bruxellensis. • Browning caused by the oxidation of hydroxycinnamic acids and key phenolic acids. • Oxidation of aroma producing thiols rendering them non volatile. To prevent the oxidation of these phenolic compounds, an anti-oxidative mechanism needs to be put in place. -

The Path to Bottling
The Path to Bottling www.laffort.com YOUR ACCESS TO 120 YEARS OF WINEMAKING INNOVATION Save it in your favourites now for 24/7 access to ! PRODUCT INFORMATION SHEETS QUALITY DOCUMENTS ( HACCP & ISO ) PRODUCT SAFETY SHEETS RESEARCH PAPERS DECISION MAKING TOOLS CATALOGUE DOWNLOADS PERSONALISED NUTRITION PUBLISHED ARTICLES CALCULATOR CERTIFICATES OF ANALYSIS ORGANIC CERTIFICATIONS TRAINING VIDEOS LAFFORT® PLANT BASED INNOVATIONS LAFFORT® unrivalled technical resources is delivering the most scientifically advanced oenological solutions from plant derived products. VEGAN FRIENDLY & ORGANIC THEY’RE NOT JUST OPTIONS THEY’RE SUPERIOR SOLUTIONS These symbols are a guide to your LAFFORT® products properties. GANI TAL OR LERGE OR C E IG L N G I A E N V A L E L E S E R UIT ABLE RGEN F F R E E Organic certification bodies have different criteria for certification and products may differ from one certification body to another. Please contact your certifying agent to confirm a products organic certification. PROTECTING YOUR WHITE WINE MANAGEMENT WHITE WINE PROTECTING YOUR WHITE WINE - A TRADITIONAL APPROACH White wines are vulnerable to oxidation and microbial changes post alcoholic fermentation. Microbial and anti-oxidative control of white wines is a first step to getting wines ready for bottling and/or storage. Threats of oxygen on finished white wines; • Proliferation of acetic acid bacteria. • Proliferation of Brettanomyces bruxellensis. • Browning caused by the oxidation of hydroxycinnamic acids and key phenolic acids. • Oxidation of aroma producing thiols rendering them non volatile. To prevent the oxidation of these phenolic compounds, an anti-oxidative mechanism needs to be put in place. -

Estudio Químico-Sensorial De La Composición No Volátil De Los Vinos
TESIS DOCTORAL Título Estudio químico-sensorial de la composición no volátil de los vinos. Influencia de técnicas de aclareo en el perfil fenólico y organoléptico de los vinos Autor/es Ana Gonzalo Diago Director/es Purificación Fernández Zurbano y Marta María Inés Dizy Soto Facultad Facultad de Ciencias, Estudios Agroalimentarios e Informática Titulación Departamento Química Curso Académico 2013-2014 Estudio químico-sensorial de la composición no volátil de los vinos. Influencia de técnicas de aclareo en el perfil fenólico y organoléptico de los vinos, tesis doctoral de Ana Gonzalo Diago, dirigida por Purificación Fernández Zurbano y Marta María Inés Dizy Soto (publicada por la Universidad de La Rioja), se difunde bajo una Licencia Creative Commons Reconocimiento-NoComercial-SinObraDerivada 3.0 Unported. Permisos que vayan más allá de lo cubierto por esta licencia pueden solicitarse a los titulares del copyright. © El autor © Universidad de La Rioja, Servicio de Publicaciones, 2014 publicaciones.unirioja.es E-mail: [email protected] DEPARTAMENTO DE QUÍMICA UNIVERSIDAD DE LA RIOJA ÁREA DE QUÍMICA ANALÍTICA Estudio químico-sensorial de la composición no volátil de los vinos. Influencia de técnicas de aclareo en el perfil fenólico y organoléptico de los vinos. Memoria presentada por ANA GONZALO DIAGO para optar al grado de Doctor con Mención de Doctor Internacional Mayo 2014 Dirigida por los profesores Dra. Purificación FERNÁNDEZ ZURBANO Dra. Marta DIZY SOTO INFORME DIRECTORES PRESENTACIÓN TESIS DOCTORAL Dña. Purificación FERNÁNDEZ ZURBANO, Profesora Titular del Departamento de Química, Área Química Analítica, de la Universidad de La Rioja y Dña. Marta DIZY SOTO, Profesora Titular del Departamento de Agricultura y Alimentación, de la Universidad de La Rioja CERTIFICAN Que la presente memoria, titulada “Estudio químico-sensorial de la composición no volátil de los vinos. -

BJCP Exam Study Guide
BJCP BEER EXAM STUDY GUIDE Last Revised: December, 2017 Contributing Authors: Original document by Edward Wolfe, Scott Bickham, David Houseman, Ginger Wotring, Dave Sapsis, Peter Garofalo, Chuck Hanning. Revised 2006 by Gordon Strong and Steve Piatz. Revised 2012 by Scott Bickham and Steve Piatz. Revised 2014 by Steve Piatz Revised 2015 by Steve Piatz Revised 2017 by Scott Bickham Copyright © 1998-2017 by the authors and the BJCP CHANGE LOG January-March, 2012: revised to reflect new exam structure, no longer interim May 1, 2012: revised yeast section, corrected T/F question 99 August, 2012: removed redundant styles for question S0, revised the additional readings list, updated the judging procedure to encompass the checkboxes on the score sheet. October 2012: reworded true/false questions 2, 4, 6, 8, 13, 26, 33, 38, 39, 42, and 118. Reworded essay question T15. March 2014: removed the Exam Program description from the document, clarified the wording on question T13. October 2015: revised for the 2015 BJCP Style Guidelines. February, 2016: revised the table for the S0 question to fix typos, removed untested styles. September-October, 2017 (Scott Bickham): moved the BJCP references in Section II.B. to Section I; incorporated a study guide for the online Entrance exam in Section II; amended the rubric for written questions S0, T1, T3, T13 and T15; rewrote the Water question and converted the rubrics for each of the Technical and Brewing Process questions to have three components; simplified the wording of the written exam questions’ added -

Science Sixtyfourth64 National Conference a Platform for Progress
science sixtyfourth64 national conference a platform for progress 64th ASEV National Conference June 24–28, 2013 Portola Hotel and Monterey Conference Center Monterey, California USA TECHNICAL ABSTRACTS american society for enology and viticulture 2013 National Conference Technical Abstracts Oral Presentation Abstracts Wednesday, June 26 Enology — Flavor/Analysis ....................................................................59–62 Viticulture — Rootstocks .......................................................................63–66 Enology — Micro/Molecular Biology ....................................................67–70 Viticulture — Pests & Diseases ..............................................................71–74 Enology — Sensory/Sensory Impacts .....................................................75–78 Viticulture — Environmental Impacts ..................................................79–82 Enology — Flavor: Impact of Yeast and Bacteria ....................................83–86 Viticulture — General ...........................................................................87–90 Thursday, June 27 Enology — Wine Stability and Oxidation ..............................................91–93 Viticulture — Cultural Practices ............................................................94–96 Enology — Tannins (Part I) .................................................................97–100 Viticulture — Water Relations ...........................................................101–105 Enology — Tannins (Part II) ..............................................................106–108 -
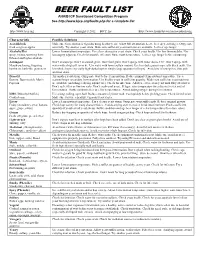
BJCP Beer Fault List
BBEEEERR FFAAUULLTT LLIISSTT AHA/BJCP Sanctioned Competition Program See http://www.bjcp.org/faults.php for a complete list http://www.bjcp.org Copyright © 2012 — BJCP, Inc. http://www.homebrewersassociation.org Characteristic Possible Solutions Acetaldehyde Make sure fermentation is vigorous using healthy yeast. Allow full attenuation. Leave beer on yeast longer. Oxygenate fresh cut green apples wort fully. Try another yeast strain. Make sure sufficient yeast nutrients are available. Let beer age longer. Alcoholic/Hot Lower fermentation temperature. Use a less attenuative yeast strain. Check yeast health. Use less fermentables. Use spicy, vinous, warming from less sugary adjuncts. Check for possible infection. Raise mash temperature. Let beer age longer before consuming. Ethanol and higher alcohols Astringent Don’t oversparge. Don’t overcrush grain. Don’t boil grain. Don’t sparge with water above 170°. Don’t sparge with Mouth-puckering, lingering water with a high pH (over 6). Use water with lower sulfate content. Use less dark grains (especially black malt). Use harshness, husk-like graininess less whole hops (especially high-alpha hops or simply large quantities of hops). Avoid use of raw spices, fruit pith and fruit skins. Diacetyl Try another yeast strain. Oxygenate wort before fermentation. Reduce primary fermentation temperature. Use a Buttery, Butterscotch, Movie warmer/longer secondary fermentation. Use healthy yeast in sufficient quantity. Make sure sufficient yeast nutrients Popcorn are available (including reducing adjunct use). Check for infection. Allow beer to rest on yeast until fully attenuated. Don’t rack, filter or fine too early. Don’t crash-cool yeast. If lager, raise temperature for a diacetyl rest at end of fermentation. -

Shropshire Tales Ad OL EMAIL
FREE Issue 8 Spring 19 Inside... Mild in May Passport Pub, Club and Brewery of the Year Results CAMRA West Midlands Regional Awards 2018 Pub and Brewery News Shropshire's Real Heritage Pubs www.tes.camra.org.uk Telford & East Shropshire CAMRA Offi cial magazine of Telford & East Shropshire and Shrewsbury & www.saws.camra.org.uk Shrewsbury & West Shropshire CAMRA West Shropshire branches of CAMRA Editor's Welcome Main Contents Hello and welcome to Issue 8 of A word from T.E.S CAMRA Chairman 4 A word from S.W.S CAMRA Chairman 4 So the votes have been cast and counted A word from our Market Drayton sub-branch 5 and we now have the results from both Pub of the Season - Spring2018 5 Shropshire branches for Pub, Club and Brewery News 7-8 Brewery of the Year for each area, see the results later in this issue. Pub News 10 CAMRA West Midlands Regional Awards 12-13 There has been some exciting news from 2018 West Midlands CAMRA as our region will TES Pub, Club & Brewery of the Year 15 be hosting the Great British Beer Festival Results Winter in Birmingham from 2020 until Mild in May 17-24 2022. This is great for us as a region SWS Pub & Club of the Year Results 26-27 and more details will be announced Notices & Emails 27 throughout the coming year. Awards presented to Shropshire pubs 28-29 Finally we are pleased to announce that & breweries Mild in May has returned to Shropshire Shropshire's Real Heritage Pubs - 30-31 this year and you can fi nd your Mild in Loggerheads, Shrewsbury May Passport in the middle of this issue Beer Festivals 2019 34 of Shropshire TAles. -

Documentation Austrian Wine 2006
DOCUMENTATION AUSTRIAN W INE 2006 Table of contents 1 Austria œ the wine country 1.1 Austria‘s wine-gr wing regi ns and wine-gr wing areas 1 1.2 Grape varieties in Austria 5 1.2.1 Breakd wn by share of area in percent 5 1.2.2 Grape varieties - Brief descripti n .1 1.2.. Devel pment f the area under cultivati n until 1111 .5 1.. Devel pment of the climate 1161-2002 .6 1.2 W ine-gr wers in Austria - A current overall view .1 1.5 The 2006 harvest 22 1.6 The 2005 vintage 25 1.3 Brief characterisati n of the vintages 2002 back t 1160 23 1.8 Assessment of the 2005-111. vintages 55 2 The Austrian wine industry 2.1 Ec n mic imp rtance of the wine industry in Austria 56 2.2 The harvest 2006 (Status Oct ber 20066 51 2.. 7arvests 1160-2005 61 2.2 8ualit9tswein (8uality wine6 in Austria 2005 65 2.5 Austria‘s wine supply 2005 68 2.6 Devel pment f grape and wine prices 31 2.3 General regulati ns f r wine pr ducti n 32 2.8 EU-Measures f r the Restructuring and C nversi n of Vineyards 32 2.1 The Austrian W ine B ards 80 2.10 The :alue f Origin 8. 2.11 DAC: the l gical key t Austrian wine 82 2.12 8uesti ns and Answers - a Guide 86 3 The Austrian market ..1 C nsumpti n of D mestic Wine and Sparkling W ine 81 ..1.1 C nsumpti n of D mestic Wine 81 ..1.2 D mestic C nsumpti n f Sparkling Wine 1110-2005 10 ..1. -

Cervejas Ruivas Para Verdadeiros Experts!
#05 quadrimestral abril ∞ julho ENTREVISTA Bruno Aquino em busca da 3.90 cultura cervejeira € LJUBOMIR STANISIC O cozinheiro que prefere cerveja artesanal 2019 . PORTUGAL CONTINENTAL 2019 . PORTUGAL CONTINENTAL TENDÊNCIAS Cerveja sem álcool conquista mercado CERVEJA E ARTE Vhils desenha coluna de cerveja CERVEJAS RUIVAS PARA VERDADEIROS EXPERTS! BOHEMIA | 1163 | MAJOR ROÇADAS | CERVETECA | PETISCARIA ST.º ANTÓNIO | PURISTA BARBIÈRE POST SCRIPTUM | LUPUM | ROTA DAS TAPAS | CERVEJÃO | MUSA | DEFEITOS: OXIDAÇÃO | NOVIDADES A TUA NOVA CERVEJA purple EDITORIAL proprietário e editor PurpleSummer Media & Events, Unip. Lda Lopes Henriques NPCpurple 513 091 378 • Capital Social € 5 000 A TUA NOVA CERVEJA Rua Manuel da Silva, n.º 2, 1º Frente 2700-552 Amadora | Portugal T. +351 211 352 336 diretora editorial Susana Marvão [email protected] T. +351 938 762 939 diretora executiva Maria Helena Duarte [email protected] T. +351 969 105 600 assessora da direção publicidade e assinaturas Cristina Ribeiro [email protected] departamento comercial Célia Borges [email protected] T. +351 912 411 317 Isabel Vieira [email protected] T. +351 962 943 695 redatores António Mendes Nunes, Aquiles Pinto, Augusto Lopes, Bruno Aquino, Carlos Ramos, Cláudia Pinto, João Pereira Santos, João Durães, SUSANA MARVÃO Manuel Baiôa, Mafalda Freire, Maria Helena Duarte, diretora editorial Paulo Pimenta, Pedro Moura, Sérgio Costa Lopes, Susana Marvão, Tito Santos fotografia Carlos Figueiredo, Ernesto Fonseca, D.R. Paixão Pela Cerveja tem o apoio da Associação Cervejeiros de Portugal impressão LusoImpress, S.A. R. Venceslau Ramos, 28 4430-929 Avintes VNG À conquista da tiragem 3000 exemplares maturidade do mercado distribuição VASP, MLP – Media Logistics Park Quinta do Grajal, Venda Seca 2739-511 Agualva Cacém Há demasiadas cervejas no mercado? À medida que vamos falando com cada vez Registo ERC | 127067 mais produtores de cerveja, a opinião parece alastrar-se não em forma de inter- Depósito Legal | 435346/17 rogação mas de ponto final. -

Potravinarstvo Slovak Journal of Food Sciences Volume 11 403 No. 1
Potravinarstvo Slovak Journal of Food Sciences Potravinarstvo Slovak Journal of Food Sciences vol. 11, 2017, no. 1, p. 403-409 doi: https://dx.doi.org/10.5219/763 Received: 20 February 2017. Accepted: 15 May 2017. Available online: 29 June 2017 at www.potravinarstvo.com © 2017 Potravinarstvo Slovak Journal of Food Sciences, License: CC BY 3.0 ISSN 1337-0960 (online) SPECIES OF GENERA BOTRYTIS, FUSARIUM AND RHIZOPUS ON GRAPES OF THE SLOVAK ORIGIN Dana Tančinová, Zuzana Mašková, Ľubormír Rybárik, Viera Michalová ABSTRACT Our research was focused to identify the Botrytis, Fusarium and Rhizopus species from grapes of the Slovak origin. A further goal of the project was to characterized toxinogenic potential of chosen strains of species Fusarium. 50 samples of grapes, harvested in years 2011, 2012 and 2013 from various wine-growing regions were analyzed in this study. For the isolation of species the of direct plating method was used: a) surface-sterilized berries (using 1% freshly pre-pared chlorine) b) berries and c) damaged berries on DRBC (Dichloran Rose Bengal Chloramphenicol agar). For each analysis were used 50 berries (or all damaged berries from sample). The cultivation was carried at 25 ±1°C, for 5 to 7 days in dark. After incubation, the colonies of Botrytis, Fusarium and Rhizopus were transferred to identification media and after incubation strains were identified to species level. Thirteen species of fusaria (F. acuminatum, F. avenaceum, F. culmorum, F. equiseti, F. graminearum, F. oxysporum, F. proliferatum, F. semitectum, F. solani, F. sporotrichioides, F. subglutinans F. tricinctum and F. verticilioides) were identified. Frequency of fusaria isolation was 92 %.