Plastic Card 1 108Fish TURKISH
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Demersal and Epibenthic Assemblages of Trawlable Grounds in the Northern Alboran Sea (Western Mediterranean)
SCIENTIA MARINA 71(3) September 2007, 513-524, Barcelona (Spain) ISSN: 0214-8358 Demersal and epibenthic assemblages of trawlable grounds in the northern Alboran Sea (western Mediterranean) ESTHER ABAD 1, IZASKUN PRECIADO 1, ALBERTO SERRANO 1 and JORGE BARO 2 1 Centro Oceanográfico de Santander, Instituto Español de Oceanografía, Promontorio de San Martín, s/n, P.O. Box 240, 39080 Santander, Spain. E-mail: [email protected] 2 Centro Oceanográfico de Málaga, Instituto Español de Oceanografía, Puerto Pesquero s/n, P.O. Box 285, 29640 Fuengirola, Málaga, Spain SUMMARY: The composition and abundance of megabenthic fauna caught by the commercial trawl fleet in the Alboran Sea were studied. A total of 28 hauls were carried out at depths ranging from 50 to 640 m. As a result of a hierarchical clas- sification analysis four assemblages were detected: (1) the outer shelf group (50-150 m), characterised by Octopus vulgaris and Cepola macrophthalma; (2) the upper slope group (151-350 m), characterised by Micromesistius poutassou, with Plesionika heterocarpus and Parapenaeus longirostris as secondary species; (3) the middle slope group (351-640 m), char- acterised by M. poutassou, Nephrops norvegicus and Caelorhincus caelorhincus, and (4) the small seamount Seco de los Olivos (310-360 m), characterised by M. poutassou, Helicolenus dactylopterus and Gadiculus argenteus, together with Chlorophthalmus agassizi, Stichopus regalis and Palinurus mauritanicus. The results also revealed significantly higher abundances in the Seco de los Olivos seamount, probably related to a higher food availability caused by strong localised cur- rents and upwellings that enhanced primary production. Although depth proved to be the main structuring factor, others such as sediment type and food availability also appeared to be important. -

Fisheries Centre
Fisheries Centre The University of British Columbia Working Paper Series Working Paper #2015 - 80 Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulman, Adib Saad, Kyrstn Zylich, Daniel Pauly and Dirk Zeller Year: 2015 Email: [email protected] This working paper is made available by the Fisheries Centre, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada. Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulmana, Adib Saadb, Kyrstn Zylicha, Daniel Paulya, Dirk Zellera a Sea Around Us, Fisheries Centre, University of British Columbia, 2202 Main Mall, Vancouver, BC, V6T 1Z4, Canada b President of Syrian National Committee for Oceanography, Tishreen University, Faculty of Agriculture, P.O. BOX; 1408, Lattakia, Syria [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected] ABSTRACT Syria’s total marine fisheries catches were estimated for the 1950-2010 time period using a reconstruction approach which accounted for all fisheries removals, including unreported commercial landings, discards, and recreational and subsistence catches. All unreported estimates were added to the official data, as reported by the Syrian Arab Republic to the United Nation’s Food and Agriculture Organization (FAO). Total reconstructed catch for 1950-2010 was around 170,000 t, which is 78% more than the amount reported by Syria to the FAO as their national catch. The unreported components added over 74,000 t of unreported catches, of which 38,600 t were artisanal landings, 16,000 t industrial landings, over 4,000 t recreational catches, 3,000 t subsistence catches and around 12,000 t were discards. -

Ecography ECOG-01937 Hattab, T., Leprieur, F., Ben Rais Lasram, F., Gravel, D., Le Loc’H, F
Ecography ECOG-01937 Hattab, T., Leprieur, F., Ben Rais Lasram, F., Gravel, D., Le Loc’h, F. and Albouy, C. 2016. Forecasting fine- scale changes in the food-web structure of coastal marine communities under climate change. – Ecography doi: 10.1111/ecog.01937 Supplementary material Forecasting fine-scale changes in the food-web structure of coastal marine communities under climate change by Hattab et al. Appendix 1 List of coastal exploited marine species considered in this study Species Genus Order Family Class Trophic guild Auxis rochei rochei (Risso, 1810) Auxis Perciformes Scombridae Actinopterygii Top predators Balistes capriscus Gmelin, 1789 Balistes Tetraodontiformes Balistidae Actinopterygii Macro-carnivorous Boops boops (Linnaeus, 1758) Boops Perciformes Sparidae Actinopterygii Basal species Carcharhinus plumbeus (Nardo, 1827) Carcharhinus Carcharhiniformes Carcharhinidae Elasmobranchii Top predators Dasyatis pastinaca (Linnaeus, 1758) Dasyatis Rajiformes Dasyatidae Elasmobranchii Top predators Dentex dentex (Linnaeus, 1758) Dentex Perciformes Sparidae Actinopterygii Macro-carnivorous Dentex maroccanus Valenciennes, 1830 Dentex Perciformes Sparidae Actinopterygii Macro-carnivorous Diplodus annularis (Linnaeus, 1758) Diplodus Perciformes Sparidae Actinopterygii Forage species Diplodus sargus sargus (Linnaeus, 1758) Diplodus Perciformes Sparidae Actinopterygii Macro-carnivorous (Geoffroy Saint- Diplodus vulgaris Hilaire, 1817) Diplodus Perciformes Sparidae Actinopterygii Basal species Engraulis encrasicolus (Linnaeus, 1758) Engraulis -

Applied Sciences
applied sciences Article Changing Isotopic Food Webs of Two Economically Important Fish in Mediterranean Coastal Lakes with Different Trophic Status Simona Sporta Caputi 1, Giulio Careddu 1 , Edoardo Calizza 1,2,*, Federico Fiorentino 1, Deborah Maccapan 1, Loreto Rossi 1,2 and Maria Letizia Costantini 1,2 1 Department of Environmental Biology, Sapienza University of Rome, via dei Sardi 70, 00185 Rome, Italy; [email protected] (S.S.C.); [email protected] (G.C.); federico.fi[email protected] (F.F.); [email protected] (D.M.); [email protected] (L.R.); [email protected] (M.L.C.) 2 CoNISMa, piazzale Flaminio 9, 00196 Rome, Italy * Correspondence: [email protected] Received: 22 February 2020; Accepted: 13 April 2020; Published: 16 April 2020 Abstract: Transitional waters are highly productive ecosystems, providing essential goods and services to the biosphere and human population. Human influence in coastal areas exposes these ecosystems to continuous internal and external disturbance. Nitrogen-loads can affect the composition of the resident community and the trophic relationships between and within species, including fish. Based on carbon (δ13C) and nitrogen (δ15N) stable isotope analyses of individuals, we explored the feeding behaviour of two ecologically and economically important omnivorous fish, the eel Anguilla anguilla and the seabream Diplodus annularis, in three neighbouring lakes characterised by different trophic conditions. We found that A. anguilla showed greater generalism in the eutrophic lake due to the increased contribution of basal resources and invertebrates to its diet. By contrast, the diet of D. annularis, which was mainly based on invertebrate species, became more specialised, focusing especially on polychaetes. -
HELCOM Red List
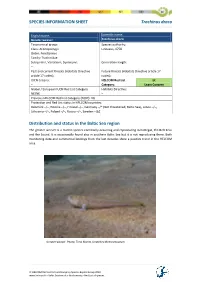
SPECIES INFORMATION SHEET Trachinus draco English name: Scientific name: Greater weever Trachinus draco Taxonomical group: Species authority: Class: Actinopterygii Linnaeus, 1758 Order: Perciformes Family: Trachinidae Subspecies, Variations, Synonyms: Generation length: – Past and current threats (Habitats Directive Future threats (Habitats Directive article 17 article 17 codes): – codes): – IUCN Criteria: HELCOM Red List LC – Category: Least Concern Global / European IUCN Red List Category Habitats Directive: NE/NE – Previous HELCOM Red List Category (2007): VU Protection and Red List status in HELCOM countries: Denmark –/–, Estonia –/–, Finland –/–, Germany –/* (Not threatened, Baltic Sea), Latvia –/–, Lithuania –/–, Poland –/–, Russia –/–, Sweden –/LC Distribution and status in the Baltic Sea region The greater weever is a marine species commonly occurring and reproducing in Kattegat, the Belt Seas and the Sound. It is occasionally found also in southern Baltic Sea but it is not reproducing there. Both monitoring data and commercial landings from the last decades show a positive trend in the HELCOM area. Greater weaver. Photo: Timo Moritz, Deutches Meeresmuseum. © HELCOM Red List Fish and Lamprey Species Expert Group 2013 www.helcom.fi > Baltic Sea trends > Biodiversity > Red List of species SPECIES INFORMATION SHEET Trachinus draco Fig.1 Catch per unit effort (number per trawling hour) of greater weever in international bottom trawl surveys in Kattegat (IBTS) during third quarter of the year (Linear fit and correlation coefficient -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Concluding Remarks
Concluding Remarks The bulk of secretory material necessary for the encapsulation of spermatozoa into a spermatophore is commonly derived from the male accessory organs of re production. Not surprisingly, the extraordinary diversity in size, shape, and struc ture of the spermatophores in different phyla is frequently a reflection of the equally spectacular variations in the anatomy and secretory performance of male reproductive tracts. Less obvious are the reasons why even within a given class or order of animals, only some species employ spermatophores, while others de pend on liquid semen as the vehicle for spermatozoa. The most plausible expla nation is that the development of methods for sperm transfer must have been in fluenced by the environment in which the animals breed, and that adaptation to habitat, rather than phylogeny, has played a decisive role. Reproduction in the giant octopus of the North Pacific, on which our attention has been focussed, pro vides an interesting example. During copulation in seawater, which may last 2 h, the sperm mass has to be pushed over the distance of 1 m separating the male and female genital orifices. The metre-long tubular spermatophore inside which the spermatozoa are conveyed offers an obvious advantage over liquid semen, which could hardly be hauled over such a long distance. Apart from acting as a convenient transport vehicle, the spermatophore serves other purposes. Its gustatory and aphrodisiac attributes, the provision of an ef fective barrier to reinsemination, and stimulation of oogenesis and oviposition are all of great importance. Absolutely essential is its function as storage organ for spermatozoa, at least as effective as that of the epididymis for mammalian spermatozoa. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Marine Invertebrate Diversity in Aristotle's Zoology
Contributions to Zoology, 76 (2) 103-120 (2007) Marine invertebrate diversity in Aristotle’s zoology Eleni Voultsiadou1, Dimitris Vafi dis2 1 Department of Zoology, School of Biology, Aristotle University of Thessaloniki, GR - 54124 Thessaloniki, Greece, [email protected]; 2 Department of Ichthyology and Aquatic Environment, School of Agricultural Sciences, Uni- versity of Thessaly, 38446 Nea Ionia, Magnesia, Greece, dvafi [email protected] Key words: Animals in antiquity, Greece, Aegean Sea Abstract Introduction The aim of this paper is to bring to light Aristotle’s knowledge Aristotle was the one who created the idea of a general of marine invertebrate diversity as this has been recorded in his scientifi c investigation of living things. Moreover he works 25 centuries ago, and set it against current knowledge. The created the science of biology and the philosophy of analysis of information derived from a thorough study of his biology, while his animal studies profoundly infl uenced zoological writings revealed 866 records related to animals cur- rently classifi ed as marine invertebrates. These records corre- the origins of modern biology (Lennox, 2001a). His sponded to 94 different animal names or descriptive phrases which biological writings, constituting over 25% of the surviv- were assigned to 85 current marine invertebrate taxa, mostly ing Aristotelian corpus, have happily been the subject (58%) at the species level. A detailed, annotated catalogue of all of an increasing amount of attention lately, since both marine anhaima (a = without, haima = blood) appearing in Ar- philosophers and biologists believe that they might help istotle’s zoological works was constructed and several older in the understanding of other important issues of his confusions were clarifi ed. -

Atlas of North Sea Fishes
ICES COOPERATIVE RESEARCH REPORT RAPPORT DES RECHERCHES COLLECTIVES NO. 194 Atlas of North Sea Fishes Based on bottom-trawl survey data for the years 1985—1987 Ruud J. Knijn1, Trevor W. Boon2, Henk J. L. Heessen1, and John R. G. Hislop3 'Netherlands Institute for Fisheries Research, Haringkade 1, PO Box 6 8 , 1970 AB Umuiden, The Netherlands 2MAFF, Fisheries Laboratory, Lowestoft, Suffolk NR33 OHT, England 3Marine Laboratory, PO Box 101, Victoria Road, Aberdeen AB9 8 DB, Scotland Fish illustrations by Peter Stebbing International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer Palægade 2—4, DK-1261 Copenhagen K, Denmark September 1993 Copyright ® 1993 All rights reserved No part of this book may be reproduced in any form by photostat or microfilm or stored in a storage system or retrieval system or by any other means without written permission from the authors and the International Council for the Exploration of the Sea Illustrations ® 1993 Peter Stebbing Published with financial support from the Directorate-General for Fisheries, AIR Programme, of the Commission of the European Communities ICES Cooperative Research Report No. 194 Atlas of North Sea Fishes ISSN 1017-6195 Printed in Denmark Contents 1. Introduction............................................................................................................... 1 2. Recruit surveys.................................................................................. 3 2.1 General purpose of the surveys..................................................................... -

Marine Fishes from Galicia (NW Spain): an Updated Checklist
1 2 Marine fishes from Galicia (NW Spain): an updated checklist 3 4 5 RAFAEL BAÑON1, DAVID VILLEGAS-RÍOS2, ALBERTO SERRANO3, 6 GONZALO MUCIENTES2,4 & JUAN CARLOS ARRONTE3 7 8 9 10 1 Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca 11 e Asuntos Marítimos, Rúa do Valiño 63-65, 15703 Santiago de Compostela, Spain. E- 12 mail: [email protected] 13 2 CSIC. Instituto de Investigaciones Marinas. Eduardo Cabello 6, 36208 Vigo 14 (Pontevedra), Spain. E-mail: [email protected] (D. V-R); [email protected] 15 (G.M.). 16 3 Instituto Español de Oceanografía, C.O. de Santander, Santander, Spain. E-mail: 17 [email protected] (A.S); [email protected] (J.-C. A). 18 4Centro Tecnológico del Mar, CETMAR. Eduardo Cabello s.n., 36208. Vigo 19 (Pontevedra), Spain. 20 21 Abstract 22 23 An annotated checklist of the marine fishes from Galician waters is presented. The list 24 is based on historical literature records and new revisions. The ichthyofauna list is 25 composed by 397 species very diversified in 2 superclass, 3 class, 35 orders, 139 1 1 families and 288 genus. The order Perciformes is the most diverse one with 37 families, 2 91 genus and 135 species. Gobiidae (19 species) and Sparidae (19 species) are the 3 richest families. Biogeographically, the Lusitanian group includes 203 species (51.1%), 4 followed by 149 species of the Atlantic (37.5%), then 28 of the Boreal (7.1%), and 17 5 of the African (4.3%) groups. We have recognized 41 new records, and 3 other records 6 have been identified as doubtful. -

Phospholipids in Mediterranean Cephalopods Vassilia J
Phospholipids in Mediterranean Cephalopods Vassilia J. Sinanoglou and Sofia Miniadis-Meimaroglou* Food Chemistry Laboratory, Department of Chemistry, University of Athens, Panepistimiopolis Zographou, 15771 Athens, Greece. Fax: (301) 7228815; (301) 7483415. E-mail: [email protected] * Author for correspondence and reprint requests Z. Naturforsch. 55c, 245-255 (2000); received September 29/November 2, 1999 Cephalopods, Octopus Lipids, Phospholipids Polar lipids of the cephalopods Eledone moschata, Sepia officinalis and Todarodes sagittatus mantle, represent 50.5%, 66.1% and 74.2% of wet tissue respectively. On the other hand the polar lipids of these three species of cephalopods constitute of 80.8%, 94.8% and 93.7% of phospholipids, respectively. The main phospholipids identified were phosphatidylcholine (52.2, 51.3 and 58.4% of total phospholipids respectively in the above mentioned species), phosphatidylethanolamine (18.1, 19.7 and 23.9%), sphingomyelin (10.7, 15.2 and 6.7%), lyso- phosphatidylcholine (3.1, 3.8 and 1.8%) and the unusual lipid ceramide aminoethylphospho- nic acid (15.9, 10 and 9.2%). The 56.8% of phosphatidylcholine in Eledone moschata, the 46% in Sepia officinalis and the 74.1% in Todarodes sagittatus refer to the structure of 1,2-diacyl-glycerocholine and the remaining percentage refer to the structure of l-oalkyl-2-acyl-glycerocholine or l-o-alkyl-l- enyl-2-acyl-glycerocholine. The 87.2% of phosphatidylethanolamine in Eledone moschata , the 81% in Sepia officinalis and the 90.7% in Todarodes sagittatus refer to the structure of 1,2-diacyl-glyceroethanolamine and the remaining percentage refer to the structure of l-o-alkyl-2-acyl-glyceroethanolamine or l-oalkyl-l-enyl-2-acyl-glyceroethanolamine.