Appendix B: Kentucky Timeline for Disease Reporting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -
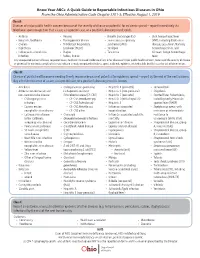
Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Melioidosis in Northern Tanzania: an Important Cause of Febrile Illness?
Melioidosis and serological evidence of exposure to Burkholderia pseudomallei among patients with fever, northern Tanzania Michael Maze Department of Medicine University of Otago, Christchurch Melioidosis Melioidosis caused by Burkholderia pseudomallei • Challenging to identify when cultured Soil reservoir, with human infection from contact with contaminated water Most infected people are asymptomatic: 1 clinical illness for 4,500 antibody producing exposures Febrile illness with a variety of presentations and bacteraemia is present in 40-60% of people with acute illness Estimated 89,000 deaths globally Gavin Koh CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=4975784 Laboratory diagnosis of febrile inpatients, northern Tanzania, 2007-8 (n=870) Malaria (1.6%) Bacteremia (9.8%) Mycobacteremia (1.6%) Fungemia (2.9%) Brucellosis (3.5%) Leptospirosis (8.8%) Q fever (5.0%) No diagnosis (50.1%) Spotted fever group rickettsiosis (8.0%) Typhus group rickettsiosis (0.4%) Chikungunya (7.9%) Crump PLoS Neglect Trop Dis 2013; 7: e2324 Predicted environmental suitability for B. pseudomallei in East Africa Large areas of Africa are predicted to be highly suitable for Burkholderia pseudomallei East Africa is less suitable but pockets of higher suitability including northern Tanzania Northern Tanzania Increasing suitability for B. pseudomallei Limmathurotsakul Nat Microbiol. 2016;1(1). Melioidosis epidemiology in Africa Paucity of empiric data • Scattered case reports • Report from Kilifi, Kenya identified 4 bacteraemic cases from 66,000 patients who had blood cultured1 • 5.9% seroprevalence among healthy adults in Uganda2 • No reports from Tanzania 1. Limmathurotsakul Nat Microbiol. 2016;1(1). 2. Frazer J R Army Med Corps. 1982;128(3):123-30. -

Taxonomy of the Order Bunyavirales: Update 2019
Archives of Virology (2019) 164:1949–1965 https://doi.org/10.1007/s00705-019-04253-6 VIROLOGY DIVISION NEWS Taxonomy of the order Bunyavirales: update 2019 Abulikemu Abudurexiti1 · Scott Adkins2 · Daniela Alioto3 · Sergey V. Alkhovsky4 · Tatjana Avšič‑Županc5 · Matthew J. Ballinger6 · Dennis A. Bente7 · Martin Beer8 · Éric Bergeron9 · Carol D. Blair10 · Thomas Briese11 · Michael J. Buchmeier12 · Felicity J. Burt13 · Charles H. Calisher10 · Chénchén Cháng14 · Rémi N. Charrel15 · Il Ryong Choi16 · J. Christopher S. Clegg17 · Juan Carlos de la Torre18 · Xavier de Lamballerie15 · Fēi Dèng19 · Francesco Di Serio20 · Michele Digiaro21 · Michael A. Drebot22 · Xiaˇoméi Duàn14 · Hideki Ebihara23 · Toufc Elbeaino21 · Koray Ergünay24 · Charles F. Fulhorst7 · Aura R. Garrison25 · George Fú Gāo26 · Jean‑Paul J. Gonzalez27 · Martin H. Groschup28 · Stephan Günther29 · Anne‑Lise Haenni30 · Roy A. Hall31 · Jussi Hepojoki32,33 · Roger Hewson34 · Zhìhóng Hú19 · Holly R. Hughes35 · Miranda Gilda Jonson36 · Sandra Junglen37,38 · Boris Klempa39 · Jonas Klingström40 · Chūn Kòu14 · Lies Laenen41,42 · Amy J. Lambert35 · Stanley A. Langevin43 · Dan Liu44 · Igor S. Lukashevich45 · Tāo Luò1 · Chuánwèi Lüˇ 19 · Piet Maes41 · William Marciel de Souza46 · Marco Marklewitz37,38 · Giovanni P. Martelli47 · Keita Matsuno48,49 · Nicole Mielke‑Ehret50 · Maria Minutolo3 · Ali Mirazimi51 · Abulimiti Moming14 · Hans‑Peter Mühlbach50 · Rayapati Naidu52 · Beatriz Navarro20 · Márcio Roberto Teixeira Nunes53 · Gustavo Palacios25 · Anna Papa54 · Alex Pauvolid‑Corrêa55 · Janusz T. Pawęska56,57 · Jié Qiáo19 · Sheli R. Radoshitzky25 · Renato O. Resende58 · Víctor Romanowski59 · Amadou Alpha Sall60 · Maria S. Salvato61 · Takahide Sasaya62 · Shū Shěn19 · Xiǎohóng Shí63 · Yukio Shirako64 · Peter Simmonds65 · Manuela Sironi66 · Jin‑Won Song67 · Jessica R. Spengler9 · Mark D. Stenglein68 · Zhèngyuán Sū19 · Sùróng Sūn14 · Shuāng Táng19 · Massimo Turina69 · Bó Wáng19 · Chéng Wáng1 · Huálín Wáng19 · Jūn Wáng19 · Tàiyún Wèi70 · Anna E. -

Notification Requirements
Protocol for Public Health Agencies to Notify CDC about the Occurrence of Nationally Notifiable Conditions, 2021 Categorized by Notification Timeliness IMMEDIATELY NOTIFIABLE, EXTREMELY URGENT: Call the CDC ROUTINELY NOTIFIABLE: Submit electronic case notification Emergency Operations Center (EOC) at 770.488.7100 within 4 hours of within the next reporting cycle. a case meeting the notification criteria, followed by submission of an electronic case notification to CDC by the next business day. IMMEDIATELY NOTIFIABLE, URGENT: Call the CDC EOC at 770.488.7100 Approved by CSTE: June 2019 within 24 hours of a case meeting the notification criteria, followed by Interim Update Approved by CSTE: April 5, 2020 submission of an electronic case notification in next regularly scheduled Implemented: January 1, 2020 electronic transmission. Updated: May 28, 2020 Condition Notification Timeliness Cases Requiring Notification Anthrax Immediately notifiable, Confirmed and probable cases - Source of infection not recognized extremely urgent - Recognized BT exposure/potential mass exposure - Serious illness of naturally-occurring anthrax Botulism Immediately notifiable, All cases prior to classification - Foodborne (except endemic to Alaska) extremely urgent - Intentional or suspected intentional release - Infant botulism (clusters or outbreaks) - Cases of unknown etiology/not meeting standard notification criteria Page 1 of 5 Plague Immediately notifiable, All cases prior to classification - Suspected intentional release extremely urgent Paralytic poliomyelitis -

Tick-Borne Disease Working Group 2020 Report to Congress
2nd Report Supported by the U.S. Department of Health and Human Services • Office of the Assistant Secretary for Health Tick-Borne Disease Working Group 2020 Report to Congress Information and opinions in this report do not necessarily reflect the opinions of each member of the Working Group, the U.S. Department of Health and Human Services, or any other component of the Federal government. Table of Contents Executive Summary . .1 Chapter 4: Clinical Manifestations, Appendices . 114 Diagnosis, and Diagnostics . 28 Chapter 1: Background . 4 Appendix A. Tick-Borne Disease Congressional Action ................. 8 Chapter 5: Causes, Pathogenesis, Working Group .....................114 and Pathophysiology . 44 The Tick-Borne Disease Working Group . 8 Appendix B. Tick-Borne Disease Working Chapter 6: Treatment . 51 Group Subcommittees ...............117 Second Report: Focus and Structure . 8 Chapter 7: Clinician and Public Appendix C. Acronyms and Abbreviations 126 Chapter 2: Methods of the Education, Patient Access Working Group . .10 to Care . 59 Appendix D. 21st Century Cures Act ...128 Topic Development Briefs ............ 10 Chapter 8: Epidemiology and Appendix E. Working Group Charter. .131 Surveillance . 84 Subcommittees ..................... 10 Chapter 9: Federal Inventory . 93 Appendix F. Federal Inventory Survey . 136 Federal Inventory ....................11 Chapter 10: Public Input . 98 Appendix G. References .............149 Minority Responses ................. 13 Chapter 11: Looking Forward . .103 Chapter 3: Tick Biology, Conclusion . 112 Ecology, and Control . .14 Contributions U.S. Department of Health and Human Services James J. Berger, MS, MT(ASCP), SBB B. Kaye Hayes, MPA Working Group Members David Hughes Walker, MD (Co-Chair) Adalbeto Pérez de León, DVM, MS, PhD Leigh Ann Soltysiak, MS (Co-Chair) Kevin R. -

IAP Guidelines on Rickettsial Diseases in Children
G U I D E L I N E S IAP Guidelines on Rickettsial Diseases in Children NARENDRA RATHI, *ATUL KULKARNI AND #VIJAY Y EWALE; FOR INDIAN A CADEMY OF PEDIATRICS GUIDELINES ON RICKETTSIAL DISEASES IN CHILDREN COMMITTEE From Smile Healthcare, Rehabilitation and Research Foundation, Smile Institute of Child Health, Ramdaspeth, Akola; *Department of Pediatrics, Ashwini Medical College, Solapur; and #Dr Yewale Multispeciality Hospital for Children, Navi Mumbai; for Indian Academy of Pediatrics “Guidelines on Rickettsial Diseases in Children” Committee. Correspondence to: Dr Narendra Rathi, Consultant Pediatrician, Smile Healthcare, Rehabilitation & Research Foundation, Smile Institute of Child Health, Ramdaspeth, Akola, Maharashtra, India. [email protected]. Objective: To formulate practice guidelines on rickettsial diseases in children for pediatricians across India. Justification: Rickettsial diseases are increasingly being reported from various parts of India. Due to low index of suspicion, nonspecific clinical features in early course of disease, and absence of easily available, sensitive and specific diagnostic tests, these infections are difficult to diagnose. With timely diagnosis, therapy is easy, affordable and often successful. On the other hand, in endemic areas, where healthcare workers have high index of suspicion for these infections, there is rampant and irrational use of doxycycline as a therapeutic trial in patients of undifferentiated fevers. Thus, there is a need to formulate practice guidelines regarding rickettsial diseases in children in Indian context. Process: A committee was formed for preparing guidelines on rickettsial diseases in children in June 2016. A meeting of consultative committee was held in IAP office, Mumbai and scientific content was discussed. Methodology and results were scrutinized by all members and consensus was reached. -

2018 DSHS Arbovirus Activity
Health and Human Texas Department of State Services Health Services Arbovirus Activity in Texas 2018 Surveillance Report August 2019 Texas Department of State Health Services Zoonosis Control Branch Overview Viruses transmitted by mosquitoes are referred to as arthropod-borne viruses or arboviruses. Arboviruses reported in Texas may include California (CAL) serogroup viruses, chikungunya virus (CHIKV), dengue virus (DENV), eastern equine encephalitis virus (EEEV), Saint Louis encephalitis virus (SLEV), western equine encephalitis virus (WEEV), West Nile virus (WNV), and Zika virus (ZIKV), many of which are endemic or enzootic in the state. In 2018, reported human arboviral disease cases were attributed to WNV (82%), DENV (11%), CHIKV (4%), ZIKV (2%), and CAL (1%) (Table 1). In addition, there were two cases reported as arbovirus disease cases which could not be diagnostically or epidemiologically differentiated between DENV and ZIKV. Animal infections or disease caused by WNV and SLEV were also reported during 2018. Local transmission of DENV, SLEV, and WNV was documented during 2018 (Figure 1). No reports of EEEV or WEEV were received during 2018. Table 1. Year-End Arbovirus Activity Summary, Texas, 2018 Positive Human* Arbovirus Mosquito Avian Equine TOTAL TOTAL Fever Neuroinvasive Severe Deaths PVD‡ Pools (Human) CAL 1 1 1 CHIK 7 7 7 DEN 20 20 20 SLE 2 0 2 WN 1,021 6 19 38 108 146 11 24 1,192 Zika** 4 4 TOTAL 1,023 6 19 65 109 0 178 11 24 1,226 CAL - California serogroup includes California encephalitis, Jamestown Canyon, Keystone, La Crosse, Snowshoe hare and Trivittatus viruses CHIK - Chikungunya DEN - Dengue SLE - Saint Louis encephalitis WN - West Nile ‡PVD - Presumptive viremic blood donors are people who had no symptoms at the time of donating blood through a blood collection agency, but whose blood tested positive when screened for the presence of West Nile virus or Zika virus. -

Laboratory Diagnostics of Rickettsia Infections in Denmark 2008–2015
biology Article Laboratory Diagnostics of Rickettsia Infections in Denmark 2008–2015 Susanne Schjørring 1,2, Martin Tugwell Jepsen 1,3, Camilla Adler Sørensen 3,4, Palle Valentiner-Branth 5, Bjørn Kantsø 4, Randi Føns Petersen 1,4 , Ole Skovgaard 6,* and Karen A. Krogfelt 1,3,4,6,* 1 Department of Bacteria, Parasites and Fungi, Statens Serum Institut (SSI), 2300 Copenhagen, Denmark; [email protected] (S.S.); [email protected] (M.T.J.); [email protected] (R.F.P.) 2 European Program for Public Health Microbiology Training (EUPHEM), European Centre for Disease Prevention and Control (ECDC), 27180 Solnar, Sweden 3 Scandtick Innovation, Project Group, InterReg, 551 11 Jönköping, Sweden; [email protected] 4 Virus and Microbiological Special Diagnostics, Statens Serum Institut (SSI), 2300 Copenhagen, Denmark; [email protected] 5 Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut (SSI), 2300 Copenhagen, Denmark; [email protected] 6 Department of Science and Environment, Roskilde University, 4000 Roskilde, Denmark * Correspondence: [email protected] (O.S.); [email protected] (K.A.K.) Received: 19 May 2020; Accepted: 15 June 2020; Published: 19 June 2020 Abstract: Rickettsiosis is a vector-borne disease caused by bacterial species in the genus Rickettsia. Ticks in Scandinavia are reported to be infected with Rickettsia, yet only a few Scandinavian human cases are described, and rickettsiosis is poorly understood. The aim of this study was to determine the prevalence of rickettsiosis in Denmark based on laboratory findings. We found that in the Danish individuals who tested positive for Rickettsia by serology, the majority (86%; 484/561) of the infections belonged to the spotted fever group. -

Protocol for Public Health Agencies to Notify CDC About the Occurrence of Nationally Notifiable Conditions, 2018 Categorized by Notification Timeliness
Protocol for Public Health Agencies to Notify CDC about the Occurrence of Nationally Notifiable Conditions, 2018 Categorized by Notification Timeliness IMMEDIATELY NOTIFIABLE, EXTREMELY URGENT: Call the CDC ROUTINELY NOTIFIABLE: Submit electronic case notification within Emergency Operations Center (EOC) at 770.488.7100 within 4 hours of a the next reporting cycle. case meeting the notification criteria, followed by submission of an electronic case notification to CDC by the next business day. IMMEDIATELY NOTIFIABLE, URGENT: Call the CDC EOC at 770.488.7100 within 24 hours of a case meeting the notification criteria, followed by Approved by CSTE: June 2017 submission of an electronic case notification in next regularly scheduled Implemented: January 1, 2018 electronic transmission. Updated: November 16, 2017 Condition Notification Timeliness Cases Requiring Notification Anthrax Immediately notifiable, Confirmed and probable cases - Source of infection not recognized extremely urgent - Recognized BT exposure/potential mass exposure - Serious illness of naturally-occurring anthrax Botulism Immediately notifiable, All cases prior to classification - Foodborne (except endemic to Alaska) extremely urgent - Intentional or suspected intentional release - Infant botulism (clusters or outbreaks) - Cases of unknown etiology/not meeting standard notification criteria Plague Immediately notifiable, All cases prior to classification - Suspected intentional release extremely urgent Paralytic poliomyelitis Immediately notifiable, Confirmed cases extremely -

Taxonomy of the Family Arenaviridae and the Order Bunyavirales: Update 2018
Archives of Virology https://doi.org/10.1007/s00705-018-3843-5 VIROLOGY DIVISION NEWS Taxonomy of the family Arenaviridae and the order Bunyavirales: update 2018 Piet Maes1 · Sergey V. Alkhovsky2 · Yīmíng Bào3 · Martin Beer4 · Monica Birkhead5 · Thomas Briese6 · Michael J. Buchmeier7 · Charles H. Calisher8 · Rémi N. Charrel9 · Il Ryong Choi10 · Christopher S. Clegg11 · Juan Carlos de la Torre12 · Eric Delwart13,14 · Joseph L. DeRisi15 · Patrick L. Di Bello16 · Francesco Di Serio17 · Michele Digiaro18 · Valerian V. Dolja19 · Christian Drosten20,21,22 · Tobiasz Z. Druciarek16 · Jiang Du23 · Hideki Ebihara24 · Toufc Elbeaino18 · Rose C. Gergerich16 · Amethyst N. Gillis25 · Jean‑Paul J. Gonzalez26 · Anne‑Lise Haenni27 · Jussi Hepojoki28,29 · Udo Hetzel29,30 · Thiện Hồ16 · Ní Hóng31 · Rakesh K. Jain32 · Petrus Jansen van Vuren5,33 · Qi Jin34,35 · Miranda Gilda Jonson36 · Sandra Junglen20,22 · Karen E. Keller37 · Alan Kemp5 · Anja Kipar29,30 · Nikola O. Kondov13 · Eugene V. Koonin38 · Richard Kormelink39 · Yegor Korzyukov28 · Mart Krupovic40 · Amy J. Lambert41 · Alma G. Laney42 · Matthew LeBreton43 · Igor S. Lukashevich44 · Marco Marklewitz20,22 · Wanda Markotter5,33 · Giovanni P. Martelli45 · Robert R. Martin37 · Nicole Mielke‑Ehret46 · Hans‑Peter Mühlbach46 · Beatriz Navarro17 · Terry Fei Fan Ng14 · Márcio Roberto Teixeira Nunes47,48 · Gustavo Palacios49 · Janusz T. Pawęska5,33 · Clarence J. Peters50 · Alexander Plyusnin28 · Sheli R. Radoshitzky49 · Víctor Romanowski51 · Pertteli Salmenperä28,52 · Maria S. Salvato53 · Hélène Sanfaçon54 · Takahide Sasaya55 · Connie Schmaljohn49 · Bradley S. Schneider25 · Yukio Shirako56 · Stuart Siddell57 · Tarja A. Sironen28 · Mark D. Stenglein58 · Nadia Storm5 · Harikishan Sudini59 · Robert B. Tesh48 · Ioannis E. Tzanetakis16 · Mangala Uppala59 · Olli Vapalahti28,30,60 · Nikos Vasilakis48 · Peter J. Walker61 · Guópíng Wáng31 · Lìpíng Wáng31 · Yànxiăng Wáng31 · Tàiyún Wèi62 · Michael R. -

DSHS Arbovirus Activity 061817
Arbovirus Activity in Texas 2017 Surveillance Report June 2018 Texas Department of State Health Services Infectious Disease Control Unit Zoonosis Control Branch Overview Viruses transmitted by mosquitoes are referred to as arthropod-borne viruses or arboviruses. Arboviruses reported in Texas may include California serogroup viruses (CAL), chikungunya virus (CHIKV), dengue virus (DENV), eastern equine encephalitis virus (EEEV), Saint Louis encephalitis virus (SLEV), western equine encephalitis virus (WEEV), West Nile virus (WNV), and Zika virus (ZIKV), many of which are endemic or enzootic in the state. In 2017, reported human arboviral disease cases were attributed to WNV (54%), ZIKV (22%), DENV (17%), and CHIKV (6%) (Table 1). Animal infections or disease caused by CAL, EEEV, SLEV, and WNV were also reported during 2017. Table 1. Year-End Arbovirus Activity Summary, Texas, 2017 California Serogroup Viruses California serogroup viruses (CAL) are bunyaviruses and include California encephalitis virus (CEV), Jamestown Canyon virus, Keystone virus, La Crosse virus (LACV), snowshoe hare virus, and Trivittatus virus. These viruses are maintained in a cycle between mosquito vectors and vertebrate hosts in forest habitats. In the United States (U.S.), approximately 80-100 reported cases of human neuroinvasive disease are caused by LACV each year (CDC), mostly in mid-Atlantic and southeastern states. From 2002-2016, Texas reported a total of 5 cases of human CAL disease (range: 0-3 cases/year): 1 case of CEV neuroinvasive disease and 4 cases of LACV neuroinvasive disease. In 2017, one CEV-positive mosquito pool was identified in Orange County (Figure 1); no human cases of CAL disease were reported.