Laboratory Diagnostics of Rickettsia Infections in Denmark 2008–2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -

Multiple Pruritic Papules from Lone Star Tick Larvae Bites
OBSERVATION Multiple Pruritic Papules From Lone Star Tick Larvae Bites Emily J. Fisher, MD; Jun Mo, MD; Anne W. Lucky, MD Background: Ticks are the second most common vec- was treated with permethrin cream and the lesions re- tors of human infectious diseases in the world. In addi- solved over the following 3 weeks without sequelae. The tion to their role as vectors, ticks and their larvae can also organism was later identified as the larva of Amblyomma produce primary skin manifestations. Infestation by the species, the lone star tick. larvae of ticks is not commonly recognized, with only 3 cases reported in the literature. The presence of mul- Conclusions: Multiple pruritic papules can pose a di- tiple lesions and partially burrowed 6-legged tick larvae agnostic challenge. The patient described herein had an can present a diagnostic challenge for clinicians. unusually large number of pruritic papules as well as tick larvae present on her skin. Recognition of lone star tick Observation: We describe a 51-year-old healthy woman larvae as a cause of multiple bites may be helpful in simi- who presented to our clinic with multiple erythematous lar cases. papules and partially burrowed organisms 5 days after exposure to a wooded area in southern Kentucky. She Arch Dermatol. 2006;142:491-494 ATIENTS WITH MULTIPLE PRU- acteristic clinical and diagnostic features of ritic papules that appear to be infestation by larvae of Amblyomma species bitespresentachallengetothe and may help clinicians make similar di- clinician. We present a case of agnoses in the future. a healthy 51-year-old woman whowasbittenbymultiplelarvaeofthetick, P REPORT OF A CASE Amblyomma species, most likely A ameri- canum or the lone star tick. -

Babela Massiliensis, a Representative of a Widespread Bacterial
Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae Isabelle Pagnier, Natalya Yutin, Olivier Croce, Kira S Makarova, Yuri I Wolf, Samia Benamar, Didier Raoult, Eugene V. Koonin, Bernard La Scola To cite this version: Isabelle Pagnier, Natalya Yutin, Olivier Croce, Kira S Makarova, Yuri I Wolf, et al.. Babela mas- siliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae. Biology Direct, BioMed Central, 2015, 10 (13), 10.1186/s13062-015-0043-z. hal-01217089 HAL Id: hal-01217089 https://hal-amu.archives-ouvertes.fr/hal-01217089 Submitted on 19 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Pagnier et al. Biology Direct (2015) 10:13 DOI 10.1186/s13062-015-0043-z RESEARCH Open Access Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae Isabelle Pagnier1, Natalya Yutin2, Olivier Croce1, Kira S Makarova2, Yuri I Wolf2, Samia Benamar1, Didier Raoult1, Eugene V Koonin2 and Bernard La Scola1* Abstract Background: Only a small fraction of bacteria and archaea that are identifiable by metagenomics can be grown on standard media. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -
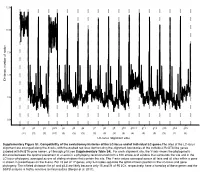
LC-Locus Alignment Sites Distance, Number of Nodes Supplementary
12.5 10.0 7.5 5.0 Distance, number of nodes 2.5 0.0 g1 g2 g3 g3.5 g4 g5 g6 g7 g8 g9 g10 g10.1 g11 g12 g13 g14 g15 (11) (3) (3) (10) (3) (3) (3) (3) (3) (3) (3) (4) (3) (3) (3) (2) (3) LC-locus alignment sites Supplementary Figure S1. Compatibility of the evolutionary histories of the LC-locus and of individual LC genes.The sites of the LC-locus alignment are arranged along the X-axis, with the dashed red lines demarcating the alignment boundaries of the individual RcGTA-like genes (labeled with RcGTA gene names, g1 through g15; see Supplementary Table S4). For each alignment site, the Y-axis shows the phylogenetic distance between the optimal placement of a taxon in a phylogeny reconstructed from a 100 amino-acid window that surrounds the site and in the LC-locus phylogeny, averaged across all sliding windows that contain the site. The Y-axis values averaged across all taxa and all sites within a gene is shown in parentheses on the X-axis. For 15 out of 17 genes, only 2-4 nodes separate the optimal taxon position in the LC-locus and gene phylogeny. The inflated distances for g1 and g3.5 are likely because only 15 and 21 of 95 LCs, respectively, have a homolog of these genes and the SSPB analysis is highly sensitive to missing data (Berger et al. 2011). a. Bacteria Unassigned Thermotogae Tenericutes Synergistetes Spirochaetes Proteobacteria ylum Planctomycetes h p Firmicutes Deferribacteres Cyanobacteria Chloroflexi Bacteroidetes Actinobacteria Acidobacteria 1(11,750) 2(1,750) 3(2,538) 4(168) 5(51) 6(54) 7(43) 8(32) 9(26) 10(40) 11(33) 12(198) 13(173) 14(101) 15(98) 16(43) 17(114) Number of rcc01682−rcc01698 homologs in a cluster b. -

Bartonella Henselae • Fleas and Black-Legged Ticks (Also Called Deer Ticks) of the Genus Ixodes May Serve As Vectors, but This Has Not Disease Agent: Been Proven
APPENDIX 2 Bartonella henselae • Fleas and black-legged ticks (also called deer ticks) of the genus Ixodes may serve as vectors, but this has not Disease Agent: been proven. • Bartonella henselae Blood Phase: Disease Agent Characteristics: • Agent found in endothelial cells and associated with RBCs in symptomatic cases • Gram-negative bacillus or coccobacillus, aerobic, • Occult bacteremia sometimes occurs in the absence nonmotile, nonspore-forming, facultatively intracel- of specific antibodies. lular bacterium • Order: Rhizobiales; Family: Bartonellaceae Survival/Persistence in Blood Products: • Size: 0.3-0.6 ¥ 0.3-1.0 mm • Nucleic acid: Approximately 1900 kb of DNA • A spiking study suggests that B. henselae added to RBCs can be recovered on solid media through 35 Disease Name: days of storage at 4°C. • Cat scratch disease • Cat scratch fever Transmission by Blood Transfusion: • Bacillary angiomatosis • Theoretical • Bacillary peliosis Cases/Frequency in Population: Priority Level: • 22,000 cases per year estimated in the US • Scientific/Epidemiologic evidence regarding blood • 2-6% in US blood donors safety: Theoretical • Cumulative seroprevalence of 7.1% to B. henselae and • Public perception and/or regulatory concern regard- B. quintana in US veterinary professionals ing blood safety: Absent • Public concern regarding disease agent: Very low Incubation Period: Background: • 3-10 days to appearance of papule at inoculation site; regional adenopathy may follow after a few weeks • In 1909,ALBartondescribed organisms that adhered to RBCs. Likelihood of Clinical Disease: • The name Bartonella bacilliformis was used for the • Relatively benign and self-limiting, lasting 6-12 weeks only member of the group identified before 1993. in the absence of antibiotic therapy • Several other species of Bartonella are known to infect humans, but at present, B. -

HIV (Human Immunodeficiency Virus)
TABLE OF CONTENTS AFRICAN TICK BITE FEVER .........................................................................................3 AMEBIASIS .....................................................................................................................4 ANTHRAX .......................................................................................................................5 ASEPTIC MENINGITIS ...................................................................................................6 BACTERIAL MENINGITIS, OTHER ................................................................................7 BOTULISM, FOODBORNE .............................................................................................8 BOTULISM, INFANT .......................................................................................................9 BOTULISM, WOUND .................................................................................................... 10 BOTULISM, OTHER ...................................................................................................... 11 BRUCELLOSIS ............................................................................................................. 12 CAMPYLOBACTERIOSIS ............................................................................................. 13 CHANCROID ................................................................................................................. 14 CHLAMYDIA TRACHOMATIS INFECTION ................................................................. -

Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Melioidosis in Northern Tanzania: an Important Cause of Febrile Illness?
Melioidosis and serological evidence of exposure to Burkholderia pseudomallei among patients with fever, northern Tanzania Michael Maze Department of Medicine University of Otago, Christchurch Melioidosis Melioidosis caused by Burkholderia pseudomallei • Challenging to identify when cultured Soil reservoir, with human infection from contact with contaminated water Most infected people are asymptomatic: 1 clinical illness for 4,500 antibody producing exposures Febrile illness with a variety of presentations and bacteraemia is present in 40-60% of people with acute illness Estimated 89,000 deaths globally Gavin Koh CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=4975784 Laboratory diagnosis of febrile inpatients, northern Tanzania, 2007-8 (n=870) Malaria (1.6%) Bacteremia (9.8%) Mycobacteremia (1.6%) Fungemia (2.9%) Brucellosis (3.5%) Leptospirosis (8.8%) Q fever (5.0%) No diagnosis (50.1%) Spotted fever group rickettsiosis (8.0%) Typhus group rickettsiosis (0.4%) Chikungunya (7.9%) Crump PLoS Neglect Trop Dis 2013; 7: e2324 Predicted environmental suitability for B. pseudomallei in East Africa Large areas of Africa are predicted to be highly suitable for Burkholderia pseudomallei East Africa is less suitable but pockets of higher suitability including northern Tanzania Northern Tanzania Increasing suitability for B. pseudomallei Limmathurotsakul Nat Microbiol. 2016;1(1). Melioidosis epidemiology in Africa Paucity of empiric data • Scattered case reports • Report from Kilifi, Kenya identified 4 bacteraemic cases from 66,000 patients who had blood cultured1 • 5.9% seroprevalence among healthy adults in Uganda2 • No reports from Tanzania 1. Limmathurotsakul Nat Microbiol. 2016;1(1). 2. Frazer J R Army Med Corps. 1982;128(3):123-30. -

Ohio Department of Health, Bureau of Infectious Diseases Disease Name Class A, Requires Immediate Phone Call to Local Health
Ohio Department of Health, Bureau of Infectious Diseases Reporting specifics for select diseases reportable by ELR Class A, requires immediate phone Susceptibilities specimen type Reportable test name (can change if Disease Name other specifics+ call to local health required* specifics~ state/federal case definition or department reporting requirements change) Culture independent diagnostic tests' (CIDT), like BioFire panel or BD MAX, E. histolytica Stain specimen = stool, bile results should be sent as E. histolytica DNA fluid, duodenal fluid, 260373001^DETECTED^SCT with E. histolytica Antigen Amebiasis (Entamoeba histolytica) No No tissue large intestine, disease/organism-specific DNA LOINC E. histolytica Antibody tissue small intestine codes OR a generic CIDT-LOINC code E. histolytica IgM with organism-specific DNA SNOMED E. histolytica IgG codes E. histolytica Total Antibody Ova and Parasite Anthrax Antibody Anthrax Antigen Anthrax EITB Acute Anthrax EITB Convalescent Anthrax Yes No Culture ELISA PCR Stain/microscopy Stain/spore ID Eastern Equine Encephalitis virus Antibody Eastern Equine Encephalitis virus IgG Antibody Eastern Equine Encephalitis virus IgM Arboviral neuroinvasive and non- Eastern Equine Encephalitis virus RNA neuroinvasive disease: Eastern equine California serogroup virus Antibody encephalitis virus disease; LaCrosse Equivocal results are accepted for all California serogroup virus IgG Antibody virus disease (other California arborviral diseases; California serogroup virus IgM Antibody specimen = blood, serum, serogroup -

Rickettsia Helvetica in Dermacentor Reticulatus Ticks
DISPATCHES The Study Rickettsia helvetica Using the cloth-dragging method, during March–May 2007 we collected 100 adult Dermacentor spp. ticks from in Dermacentor meadows in 2 different locations near Cakovec, between the Drava and Mura rivers in the central part of Medjimurje Coun- reticulatus Ticks ty. This area is situated in the northwestern part of Croatia, at Marinko Dobec, Dragutin Golubic, 46″38′N, 16″43′E, and has a continental climate with an Volga Punda-Polic, Franz Kaeppeli, average annual air temperature of 10.4°C at an altitude of and Martin Sievers 164 m. To isolate DNA from ticks, we modifi ed the method We report on the molecular evidence that Dermacentor used by Nilsson et al. (11). Before DNA isolation, ticks reticulatus ticks in Croatia are infected with Rickettsia hel- were disinfected in 70% ethanol and dried. Each tick was vetica (10%) or Rickettsia slovaca (2%) or co-infected with mechanically crushed in a Dispomix 25 tube with lysis buf- both species (1%). These fi ndings expand the knowledge of fer by using the Dispomix (Medic Tools, Zug, Switzerland). the geographic distribution of R. helvetica and D. reticulatus Lysis of each of the crushed tick samples was carried out in ticks. a solution of 6.7% sucrose, 0.2% proteinase K, 20 mg/mL lysozyme, and 10 ng/ml RNase A for 16 h at 37°C; 0.5 mo- ickettsia helvetica organisms were fi rst isolated from lar EDTA, and 20% sodium dodecyl sulfate was added and RIxodes ricinus ticks in Switzerland and were consid- further incubated for 1 h at 37°C. -

Mechanisms of Rickettsia Parkeri Invasion of Host Cells and Early Actin-Based Motility
Mechanisms of Rickettsia parkeri invasion of host cells and early actin-based motility By Shawna Reed A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Microbiology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Matthew D. Welch, Chair Professor David G. Drubin Professor Daniel Portnoy Professor Kathleen R. Ryan Fall 2012 Mechanisms of Rickettsia parkeri invasion of host cells and early actin-based motility © 2012 By Shawna Reed ABSTRACT Mechanisms of Rickettsia parkeri invasion of host cells and early actin-based motility by Shawna Reed Doctor of Philosophy in Microbiology University of California, Berkeley Professor Matthew D. Welch, Chair Rickettsiae are obligate intracellular pathogens that are transmitted to humans by arthropod vectors and cause diseases such as spotted fever and typhus. Spotted fever group (SFG) Rickettsia hijack the host actin cytoskeleton to invade, move within, and spread between eukaryotic host cells during their obligate intracellular life cycle. Rickettsia express two bacterial proteins that can activate actin polymerization: RickA activates the host actin-nucleating Arp2/3 complex while Sca2 directly nucleates actin filaments. In this thesis, I aimed to resolve which host proteins were required for invasion and intracellular motility, and to determine how the bacterial proteins RickA and Sca2 contribute to these processes. Although rickettsiae require the host cell actin cytoskeleton for invasion, the cytoskeletal proteins that mediate this process have not been completely described. To identify the host factors important during cell invasion by Rickettsia parkeri, a member of the SFG, I performed an RNAi screen targeting 105 proteins in Drosophila melanogaster S2R+ cells.