Medicago Sativa L.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inflorescences
Inflorescences - Floral Displays Inflorescences - Floral Displays A shift from widely The vast majority of flowering plants spaced single flowers to an inflorescence required possess flowers in clusters called an condensation of shoots inflorescence. and the loss of the intervening leaves. These clusters facilitate pollination via a prominent visual display and more The simplest efficient pollen uptake and deposition. inflorescence type would thus be indeterminate with the oldest flowers at the base and the younger flowers progressively closer to the apical meristem of the shoot. = a raceme Raceme (Prunus or cherry) One modification of the basic raceme is to make it compound The panicle is essentially compound a series of attached racemes with the oldest racemes at the base and the youngest at the apex of the inflorescence. Panicle (Zigadenus or white camass) Raceme Panicle 1 A second modification of the basic raceme is to lose its pedicels The spike is usually associated with congested reduced flowers and often, but not always, with wind Pedicel loss pollination. wind pollinated Spike (Plantago or plantain) Raceme Spike A third modification of the basic raceme is to lose its internodes The spike is usually associated with congested reduced flowers and often, animal pollinated but not always, with wind Internode loss pollination. Umbel Spike (Combretum - Brent’s plants) Raceme 2 The umbel characterizes specific families (carrot and The umbel is found scattered in many other families ginseng families for example). as well. These families typically show a compound umbel - smaller umbellets on a larger umbel. Umbel Umbel (Cicuta or water hemlock) (Zizia or golden alexander) (Eriogonum or false buckwheat - family Polygonaceae) - Ben’s plants A fourth modification of the basic raceme is for the stem axis to form a head The head or capitulum characterizes specific families - most notably the Compositae or Asteraceae. -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -

Introductory Grass Identification Workshop University of Houston Coastal Center 23 September 2017
Broadleaf Woodoats (Chasmanthium latifolia) Introductory Grass Identification Workshop University of Houston Coastal Center 23 September 2017 1 Introduction This 5 hour workshop is an introduction to the identification of grasses using hands- on dissection of diverse species found within the Texas middle Gulf Coast region (although most have a distribution well into the state and beyond). By the allotted time period the student should have acquired enough knowledge to identify most grass species in Texas to at least the genus level. For the sake of brevity grass physiology and reproduction will not be discussed. Materials provided: Dried specimens of grass species for each student to dissect Jewelry loupe 30x pocket glass magnifier Battery-powered, flexible USB light Dissecting tweezer and needle Rigid white paper background Handout: - Grass Plant Morphology - Types of Grass Inflorescences - Taxonomic description and habitat of each dissected species. - Key to all grass species of Texas - References - Glossary Itinerary (subject to change) 0900: Introduction and house keeping 0905: Structure of the course 0910: Identification and use of grass dissection tools 0915- 1145: Basic structure of the grass Identification terms Dissection of grass samples 1145 – 1230: Lunch 1230 - 1345: Field trip of area and collection by each student of one fresh grass species to identify back in the classroom. 1345 - 1400: Conclusion and discussion 2 Grass Structure spikelet pedicel inflorescence rachis culm collar internode ------ leaf blade leaf sheath node crown fibrous roots 3 Grass shoot. The above ground structure of the grass. Root. The below ground portion of the main axis of the grass, without leaves, nodes or internodes, and absorbing water and nutrients from the soil. -

Ornamental Garden Plants of the Guianas Pt. 2
Surinam (Pulle, 1906). 8. Gliricidia Kunth & Endlicher Unarmed, deciduous trees and shrubs. Leaves alternate, petiolate, odd-pinnate, 1- pinnate. Inflorescence an axillary, many-flowered raceme. Flowers papilionaceous; sepals united in a cupuliform, weakly 5-toothed tube; standard petal reflexed; keel incurved, the petals united. Stamens 10; 9 united by the filaments in a tube, 1 free. Fruit dehiscent, flat, narrow; seeds numerous. 1. Gliricidia sepium (Jacquin) Kunth ex Grisebach, Abhandlungen der Akademie der Wissenschaften, Gottingen 7: 52 (1857). MADRE DE CACAO (Surinam); ACACIA DES ANTILLES (French Guiana). Tree to 9 m; branches hairy when young; poisonous. Leaves with 4-8 pairs of leaflets; leaflets elliptical, acuminate, often dark-spotted or -blotched beneath, to 7 x 3 (-4) cm. Inflorescence to 15 cm. Petals pale purplish-pink, c.1.2 cm; standard petal marked with yellow from middle to base. Fruit narrowly oblong, somewhat woody, to 15 x 1.2 cm; seeds up to 11 per fruit. Range: Mexico to South America. Grown as an ornamental in the Botanic Gardens, Georgetown, Guyana (Index Seminum, 1982) and in French Guiana (de Granville, 1985). Grown as a shade tree in Surinam (Ostendorf, 1962). In tropical America this species is often interplanted with coffee and cacao trees to shade them; it is recommended for intensified utilization as a fuelwood for the humid tropics (National Academy of Sciences, 1980; Little, 1983). 9. Pterocarpus Jacquin Unarmed, nearly evergreen trees, sometimes lianas. Leaves alternate, petiolate, odd- pinnate, 1-pinnate; leaflets alternate. Inflorescence an axillary or terminal panicle or raceme. Flowers papilionaceous; sepals united in an unequally 5-toothed tube; standard and wing petals crisped (wavy); keel petals free or nearly so. -

Evolution of Flowers and Inflorescences
Development 1994 Supplement, 107-116 (1994) 107 Printed in Great Britain @ The Company of Biologists Limited 1994 Evolution of flowers and inflorescences Enrico S. Coen* and Jacqueline M. Nugent John lnnes Centre, Colney, Norwich NR4 7UH, UK *Author for correspondence SUMMARY Plant development depends on the activity of meristems of flowers. Theflo and cen genes play a key role in the posi- which continually reiterate a common plan. Permutations tioning of flowers, and variation in the site and timing of around this plan can give rise to a wide range of mor- expression of these genes, may account for many of the phologies. To understand the mechanisms underlying this different inflorescence types. The evolution of inflorescence variation, the effects of parallel mutations in key develop- stiucture may also have influenced the evolution of floral mental genes are being studied in different species. In asymmetry, as illustrated by the cen mutation which Antirrhinum, three of these key genes are: (l)floricaula ffio) changes both inflorescence type and the symmetry of some a gene required for the production of flowers (2) centror&- flowers. Conflicting theories about the origins of irregular dialis (cen), a gene controlling flower position (3) cycloidea flowers and how they have coevolved with inflorescence (cyc), a gene controlling flower symmetry. Several plant architecture can be directly assessed by examining the role species, exhibiting a range of inflorescence types and floral of cyc- and cen-like genes in species displaying various symmetries are being analysed in detail. Comparative floral symmetries and inflorescence types. genetic and molecular analysis shows that inflorescence architecture depends on two underlying parameters: a basic inflorescence branching pattern and the positioning Key words: Antirrhinum, Arabidopsis, symmetry INTRODUCTION important insights into how diverse plant forms have evolved. -
Native Plants North Georgia
Native Plants of North Georgia A photo guide for plant enthusiasts Mickey P. Cummings · The University of Georgia® · College of Agricultural and Environmental Sciences · Cooperative Extension CONTENTS Plants in this guide are arranged by bloom time, and are listed alphabetically within each bloom period. Introduction ................................................................................3 Blood Root .........................................................................5 Common Cinquefoil ...........................................................5 Robin’s-Plantain ..................................................................6 Spring Beauty .....................................................................6 Star Chickweed ..................................................................7 Toothwort ..........................................................................7 Early AprilEarly Trout Lily .............................................................................8 Blue Cohosh .......................................................................9 Carolina Silverbell ...............................................................9 Common Blue Violet .........................................................10 Doll’s Eye, White Baneberry ...............................................10 Dutchman’s Breeches ........................................................11 Dwarf Crested Iris .............................................................11 False Solomon’s Seal .........................................................12 -

PLANT MORPHOLOGY: Vegetative & Reproductive
PLANT MORPHOLOGY: Vegetative & Reproductive Study of form, shape or structure of a plant and its parts Vegetative vs. reproductive morphology http://commons.wikimedia.org/wiki/File:Peanut_plant_NSRW.jpg Vegetative morphology http://faculty.baruch.cuny.edu/jwahlert/bio1003/images/anthophyta/peanut_cotyledon.jpg Seed = starting point of plant after fertilization; a young plant in which development is arrested and the plant is dormant. Monocotyledon vs. dicotyledon cotyledon = leaf developed at 1st node of embryo (seed leaf). “Textbook” plant http://bio1903.nicerweb.com/Locked/media/ch35/35_02AngiospermStructure.jpg Stem variation Stem variation http://www2.mcdaniel.edu/Biology/botf99/stems&leaves/barrel.jpg http://www.puc.edu/Faculty/Gilbert_Muth/art0042.jpg http://www2.mcdaniel.edu/Biology/botf99/stems&leaves/xstawb.gif http://biology.uwsp.edu/courses/botlab/images/1854$.jpg Vegetative morphology Leaf variation Leaf variation Leaf variation Vegetative morphology If the primary root persists, it is called a “true root” and may take the following forms: taproot = single main root (descends vertically) with small lateral roots. fibrous roots = many divided roots of +/- equal size & thickness. http://oregonstate.edu/dept/nursery-weeds/weedspeciespage/OXALIS/oxalis_taproot.jpg adventitious roots = roots that originate from stem (or leaf tissue) rather than from the true root. All roots on monocots are adventitious. (e.g., corn and other grasses). http://plant-disease.ippc.orst.edu/plant_images/StrawberryRootLesion.JPG Root variation http://bio1903.nicerweb.com/Locked/media/ch35/35_04RootDiversity.jpg Flower variation http://130.54.82.4/members/Okuyama/yudai_e.htm Reproductive morphology: flower Yuan Yaowu Flower parts pedicel receptacle sepals petals Yuan Yaowu Flower parts Pedicel = (Latin: ped “foot”) stalk of a flower. -

Harvard Papers in Botany Volume 22, Number 1 June 2017
Harvard Papers in Botany Volume 22, Number 1 June 2017 A Publication of the Harvard University Herbaria Including The Journal of the Arnold Arboretum Arnold Arboretum Botanical Museum Farlow Herbarium Gray Herbarium Oakes Ames Orchid Herbarium ISSN: 1938-2944 Harvard Papers in Botany Initiated in 1989 Harvard Papers in Botany is a refereed journal that welcomes longer monographic and floristic accounts of plants and fungi, as well as papers concerning economic botany, systematic botany, molecular phylogenetics, the history of botany, and relevant and significant bibliographies, as well as book reviews. Harvard Papers in Botany is open to all who wish to contribute. Instructions for Authors http://huh.harvard.edu/pages/manuscript-preparation Manuscript Submission Manuscripts, including tables and figures, should be submitted via email to [email protected]. The text should be in a major word-processing program in either Microsoft Windows, Apple Macintosh, or a compatible format. Authors should include a submission checklist available at http://huh.harvard.edu/files/herbaria/files/submission-checklist.pdf Availability of Current and Back Issues Harvard Papers in Botany publishes two numbers per year, in June and December. The two numbers of volume 18, 2013 comprised the last issue distributed in printed form. Starting with volume 19, 2014, Harvard Papers in Botany became an electronic serial. It is available by subscription from volume 10, 2005 to the present via BioOne (http://www.bioone. org/). The content of the current issue is freely available at the Harvard University Herbaria & Libraries website (http://huh. harvard.edu/pdf-downloads). The content of back issues is also available from JSTOR (http://www.jstor.org/) volume 1, 1989 through volume 12, 2007 with a five-year moving wall. -
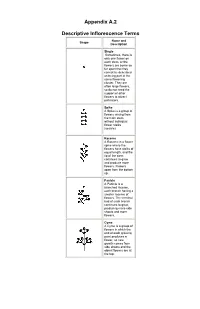
Appendix A.2 Descriptive Inflorescence Terms
Appendix A.2 Descriptive Inflorescence Terms Name and Shape Description Single Sometimes, there is only one flower on each stem, or the flowers are borne so far apart that they cannot be described as being part of the same flowering cluster. They are often large flowers, so do not need the support of other flowers to attract pollinators. Spike A Spike is a group of flowers arising from the main stem, without individual flower stalks (sessile). Raceme A Raceme is a flower spike where the flowers have stalks of equal length, and the tip of the stem continues to grow and produce more flowers. Flowers open from the bottom up. Panicle A Panicle is a branched raceme, each branch having a smaller raceme of flowers. The terminal bud of each branch continues to grow, producing more side shoots and more flowers. Cyme A Cyme is a group of flowers in which the end of each growing point produces a flower, so new growth comes from side shoots and the oldest flowers are at the top. Verticillaster A Verticillaster is a whorled inflorescence, where the flowers are borne in rings at intervals up the stem. The tip continues to grow, producing more whorls. This type of inflorescence is common in members of the Deadnettle/Mint Family (Lamiaceae). Corymb A Corymb is a flower cluster where all the flowers are at the same level, with flower stalks of different lengths, forming a flat-topped flower cluster. Umbel An Umbel is a flower head in which all the flower stalks are of the same length, so that the flower head is rounded like an umbrella. -

Dictionary of Cultivated Plants and Their Regions of Diversity Second Edition Revised Of: A.C
Dictionary of cultivated plants and their regions of diversity Second edition revised of: A.C. Zeven and P.M. Zhukovsky, 1975, Dictionary of cultivated plants and their centres of diversity 'N -'\:K 1~ Li Dictionary of cultivated plants and their regions of diversity Excluding most ornamentals, forest trees and lower plants A.C. Zeven andJ.M.J, de Wet K pudoc Centre for Agricultural Publishing and Documentation Wageningen - 1982 ~T—^/-/- /+<>?- •/ CIP-GEGEVENS Zeven, A.C. Dictionary ofcultivate d plants andthei rregion so f diversity: excluding mostornamentals ,fores t treesan d lowerplant s/ A.C .Zeve n andJ.M.J ,d eWet .- Wageninge n : Pudoc. -11 1 Herz,uitg . van:Dictionar y of cultivatedplant s andthei r centreso fdiversit y /A.C .Zeve n andP.M . Zhukovsky, 1975.- Me t index,lit .opg . ISBN 90-220-0785-5 SISO63 2UD C63 3 Trefw.:plantenteelt . ISBN 90-220-0785-5 ©Centre forAgricultura l Publishing and Documentation, Wageningen,1982 . Nopar t of thisboo k mayb e reproduced andpublishe d in any form,b y print, photoprint,microfil m or any othermean swithou t written permission from thepublisher . Contents Preface 7 History of thewor k 8 Origins of agriculture anddomesticatio n ofplant s Cradles of agriculture and regions of diversity 21 1 Chinese-Japanese Region 32 2 Indochinese-IndonesianRegio n 48 3 Australian Region 65 4 Hindustani Region 70 5 Central AsianRegio n 81 6 NearEaster n Region 87 7 Mediterranean Region 103 8 African Region 121 9 European-Siberian Region 148 10 South American Region 164 11 CentralAmerica n andMexica n Region 185 12 NorthAmerica n Region 199 Specieswithou t an identified region 207 References 209 Indexo fbotanica l names 228 Preface The aimo f thiswor k ist ogiv e thereade r quick reference toth e regionso f diversity ofcultivate d plants.Fo r important crops,region so fdiversit y of related wild species areals opresented .Wil d species areofte nusefu l sources of genes to improve thevalu eo fcrops . -

Plant Anatomy & Plant Parts
Flower and Inflorescence Types What you need to know to identify plants Wildland Plant Identification REM 252 Flowers and Reproduction New Plant Flower Fruit/Seed Pollination Structure of Flowers Structure of Flowers PERFECT Flower = includes stamens (♂ or male) AND pistil (♀ or female) IMPERFECT Flower = has stamens (♂ or male) OR pistil (♀ or female) but not both Perfect Flowers Imperfect Flowers Monoecious - ♂ and ♀ flowers on SAME plant Dioecious - ♂ and ♀ flowers on SEPARATE plants Monoecious Dioecious Male Male Plant Female Male Female Female Plant Imperfect Flowers Monoecious - ♂ and ♀ flowers on SAME plant Dioecious - ♂ and ♀ flowers on SEPARATE plants Monoecious Dioecious Male Plant Male Female Female Plant Structure of Flowers Flower Inflorescence Structure of Flowers Inflorescence - Group of flowers Structure of Flowers Catkin = a dense spike or raceme with many small, usually naked, flowers. Cyme = a convex or flat-topped flower cluster with the central flower the first to open. A determinate inflorescence. Panicle Flowers attached to branching branches Raceme Flowers attached to main stalk with pedicels Spike Flowers connected directly to main stalk (not on panicle branches off main stem) * You do not need to memorize the terms pedicel or sessile Umbel All pedicles appx. same length connect to stalk at same location This is a compound umbel (umbel with umbels at the tips) Corymb Raceme with longer (uneven) pedicles at bottom of inflorescence Flowers appear to be at the same height Corymb = a simple racemose inflorescence that is flat-topped. An indeterminate inflorescence. Cyme Oldest flower in center. Younger flowers grow on outside with longer pedicles so flowers appear to be at the same height. -

Glossary of Botanical Terms Used in the Poaceae Adapted from the Glossary in Flora of Ethiopia and Eritrea, Vol
Glossary of botanical terms used in the Poaceae Adapted from the glossary in Flora of Ethiopia and Eritrea, vol. 7 (1995). aristate – with an awn lodicule – a small scale-like or fleshy structure at the base of the sta- aristulate – diminutive of aristate mens in a grass floret, usually 2 in each floret (often 3 or more in auricle – an earlike lobe or appendage at the junction of leaf sheath and bamboos); they swell at anthesis, causing the floret to gape open blade oral setae – marginal setae inserted at junction of leaf sheath and blade, auriculate – with an auricle on the auricles when these are present awn – a bristle arising from a spikelet part pachymorph (bamboos) – rhizome sympodial, thicker than culms callus – a hard projection at the base of a floret, spikelet, or inflores- palea – the upper and inner scale of the grass floret which encloses the cence segment, indicating a disarticulation point grass flower, usually 2-keeled caryopsis – a specialized dry fruit characteristic of grasses, in which the panicle – in grasses, an inflorescence in which the primary axis bears seed and ovary wall have become united branched secondary axes with pedicellate spikelets collar – pale or purplish zone at the junction of leaf sheath and blade pedicel – in grasses, the stalk of a single spikelet within an inflo- rescence column – the lower twisted portion of a geniculate awn, or the part be- low the awn branching-point in Aristideae peduncle – the stalk of a raceme or cluster of spikelets compound – referring to inflorescences made up of a