Identifying Choke Cherry a Source of X Disease Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inflorescences
Inflorescences - Floral Displays Inflorescences - Floral Displays A shift from widely The vast majority of flowering plants spaced single flowers to an inflorescence required possess flowers in clusters called an condensation of shoots inflorescence. and the loss of the intervening leaves. These clusters facilitate pollination via a prominent visual display and more The simplest efficient pollen uptake and deposition. inflorescence type would thus be indeterminate with the oldest flowers at the base and the younger flowers progressively closer to the apical meristem of the shoot. = a raceme Raceme (Prunus or cherry) One modification of the basic raceme is to make it compound The panicle is essentially compound a series of attached racemes with the oldest racemes at the base and the youngest at the apex of the inflorescence. Panicle (Zigadenus or white camass) Raceme Panicle 1 A second modification of the basic raceme is to lose its pedicels The spike is usually associated with congested reduced flowers and often, but not always, with wind Pedicel loss pollination. wind pollinated Spike (Plantago or plantain) Raceme Spike A third modification of the basic raceme is to lose its internodes The spike is usually associated with congested reduced flowers and often, animal pollinated but not always, with wind Internode loss pollination. Umbel Spike (Combretum - Brent’s plants) Raceme 2 The umbel characterizes specific families (carrot and The umbel is found scattered in many other families ginseng families for example). as well. These families typically show a compound umbel - smaller umbellets on a larger umbel. Umbel Umbel (Cicuta or water hemlock) (Zizia or golden alexander) (Eriogonum or false buckwheat - family Polygonaceae) - Ben’s plants A fourth modification of the basic raceme is for the stem axis to form a head The head or capitulum characterizes specific families - most notably the Compositae or Asteraceae. -

Black Cherry (Prunus Serotina Ehrh.)
DOCS A 13.31:C 424/971 .: z,' BLACK CHERRY Black cherry, also commonly called cherry, grows in significant commercial quantities only in the north- ern Allegheny Mountains. Cherry wood is reddish and takes a lustrous finish; it is a prized furniture wood and brings high prices in veneer log form. lt is straight-grained moderately hard, and stable; it can be machined easily. Black cherry is widely used in the printing industries to mount engravings, elec- trotypes, and zinc etchings. lt is also used for wall paneling, flooring, patterns, professional and scien- tific instruments, handles, and other specialty items. -"3242, /9 \(j\\ '-' 'Li \c1 - - / U.S. Department of Agriculture Forest Service . American Woods-FS 229 Revised February 1971 BLACK CHERRY (Prunus sero tina Ehrh.) Charles J. Gatchell1 DISTRiBUTION Florida west to eastern Texas, north to central Minne. z Black cherry and its varieties grow under a wide sota, and east through northern Michigan, Ontario, range of climatic conditions (fig. 1). It is found prin. and Quebec to Maine and Nova Scotia. It is also found cipally throughout the eastern half of the United States, in scattered locations in Arizona, New Mexico, western from the Plains to the Atlantic, and the Great Lakes to Texas, Guatemala, and Mexico. It grows extensively in the Gulf of Mexico. Its range extends from northern western and central Mexico. Research forest products technologist, Northeastern Forest Black cherry is of commercial significance only in Experiment Station, USDA Forest Service, a narrow area centering in western Pennsylvania. Major 0 ?_ .90.?0 3?0. 95 F-506642 Figure 1.-Natural range of black cherry, Prunus serotina. -

Pollination of Cultivated Plants in the Tropics 111 Rrun.-Co Lcfcnow!Cdgmencle
ISSN 1010-1365 0 AGRICULTURAL Pollination of SERVICES cultivated plants BUL IN in the tropics 118 Food and Agriculture Organization of the United Nations FAO 6-lina AGRICULTUTZ4U. ionof SERNES cultivated plans in tetropics Edited by David W. Roubik Smithsonian Tropical Research Institute Balboa, Panama Food and Agriculture Organization of the United Nations F'Ø Rome, 1995 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-11 ISBN 92-5-103659-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. FAO 1995 PlELi. uion are ted PlauAr David W. Roubilli (edita Footli-anal ISgt-iieulture Organization of the Untled Nations Contributors Marco Accorti Makhdzir Mardan Istituto Sperimentale per la Zoologia Agraria Universiti Pertanian Malaysia Cascine del Ricci° Malaysian Bee Research Development Team 50125 Firenze, Italy 43400 Serdang, Selangor, Malaysia Stephen L. Buchmann John K. S. Mbaya United States Department of Agriculture National Beekeeping Station Carl Hayden Bee Research Center P. -

A Study of the Pollination of the Sour Cherry, Prunus Cerasus Linnaeus
THE S IS on A STUDY OF THE POLLINATION OF THE SOUR CHERRY PRTJNIJS ERASUS L INNAEUS Submitted to the OREGON AGRICULTURAL COLLEGE In Partial Fulfillment of the Require!rßnte For the Degree of MASTER OF SCIENCE by Loue Arrowood 1etchor May 5, 126. PRQYO: Redacted for privacy £eoc1at ProfEor of In ohare of Major Redacted for privacy 4-.----- - - - - 'j Road of Dopartnent of Redacted for privacy of Redacted for privacy atzn of comi.ttee on Graivate Study. III QNQLEDGE lIE NT The writer wishes to express hie appreciation to Dr. E. M. Harvey of the Research Division, for hie untiring help and many suggestion. which aided greatly in carrying out the following prob- leì; and to Prcfesaor C. E. Schuster, for his critciems and timely suggestions on the field work; and to Mr. R. V. Rogers of Eugene, for use of his trees; and to Professor J. S. Brown, who made this problem poasble. Iv - INDEX- Page. Title Page I Approval Sheet II Acknowledgment III Index IV List of Table. V List of Plates VI Introduction i Review of Literature 3 Methods and Materials 10 Germination Tests 12 Preliminary Survey of Work 14 Sterility Tests 15 Cross Pollination Studies 20 Dicusiion 28 Si.ary 29 Histological Studies 31 Methode and Materials A - Bud Development Studies 31 B - Pieti]. Studies 33 Methods and Materials 33 Diecuseion of Results A - Bud Development Studies 35 B - Pistil Studies 37 Swary 38 Explanation of Plates 39 Platos 42 BiblIography 47 V -LIST OF TABLES- Table No. Pag. I Germination Teats 13 II Sterility Test. -

Ships of 8 Tree Species in Navajo National Monument, Arizona
Population Dynamics and Age Relation- ships of 8 Tree Species in Navajo National Monument, Arizona J.D. BROTHERSON, S.R. RUSHFORTH, W.E. EVENSON, J.R. JOHANSEN, AND C. MORDEN The presence of 3 major archeological ruins dating from the 1 lth cent slickrock areas and the plateau behind the canyon rim to 13th centuries (Woodbury 1963) provided the primary motiva- (Brotherson et al. 1978). The pigmy woodland community covers tion for including Navajo National Monument in the National more area than any other type in northeastern Arizona. Parks System. Also included in the monument are some unique Gambel oak is the most extensive type in the Monument aside ecosystems, especially a small relict of “mountain vegetation” from tne pinyon-juniper community. It is found inall 3 segments of found in Betatakin Canyon. As visitor pressures mount annually, the monument but reaches its greatest development at Keet Steel proper management of these unique ecosystems becomes highly and Betatakin. Pnrnus emarginata (bittercherry) also grows at important. Since trees are the dominant features of these ecosys- Keet Steel but is uncommon. tems, and are central to management considerations, the present The streamside community of the Inscription House segment of work has examined populations of 8 major tree species in the the monument contains Popufus fremontii (Fremont poplar), monument. Populus angustifolia (narrowleaf cottonwood), Salk goodingii The objectives of this study were, first, to develop age prediction (Gooding willow), Tamarix ramosissima (salt cedar), and Elaeg- equations for tree species growing in Navajo National Monument, nus angustifolia (Russian olive). Fremont poplar is the dominant and second, to assess the present age profiles, reproductive recruit- tree within this community with salt cedar attaining local impor- ment, and density relationships of these tree populations. -

X-Disease in Peaches 44
X-disease pathogen Candidatus Phytoplasma pruni. Hosts Sweet and tart cherry, peach and nectarine. Alternate hosts include clovers, dandelion, chokecherry, almond and several wild plum and cherry species. Time of concern Management is focused on removing infected hosts before leafhopper spread can occur. Symptoms and damage William Shane, MSU Extension Peach and nectarine leaves develop red, necrotic Sweet cherry leaf with enlarged leaf-like stipules at the base of the areas that drop out, leaving a shot-hole effect and petiole due to X-disease. tattered leaves. Defoliation at the base of a shoot gives a poodle tail or pompom appearance. Fruit on infected branches is smaller, lacks flavor often with a bitter taste and may drop before ripening. Usually by the third year after infection, most branches will show symptoms. Young trees die within one to two years after the first symptoms appear. Older trees gradually decline in vigor. Sweet and tart cherries infected with X-disease phy- toplasma show stunted growth, enlarged stipules and immature, small, poorly colored fruit. Infected cherry trees on mahaleb rootstock decline quickly, whereas those on mazzard rootstock may persist for many years. Pest cycle Chokecherry is an important natural reservoir of peach X-disease phytoplasma in eastern USA. Infected sweet and sour cherries, especially on maz- zard rootstock, can be sources of X-disease, although chokecherry is often the principal reservoir. Other reservoirs of X-disease include weeds such as clover and dandelion. Peaches and nectarines, although severely affected William Shane, MSU Extension by the pathogen, are poor hosts for further disease Peach leaf with wine-red splotches typical of X-disease symptoms. -

Comparing Negative Impacts of Prunus Serotina, Quercus Rubra and Robinia Pseudoacacia on Native Forest Ecosystems
Article Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems Rodolfo Gentili 1,* , Chiara Ferrè 1, Elisa Cardarelli 2, Chiara Montagnani 1, Giuseppe Bogliani 2 , Sandra Citterio 1 and Roberto Comolli 1 1 Department of Earth and Environmental Sciences, University of Milano-Bicocca, Piazza della Scienza 1, 20126 Milano, Italy; [email protected] (C.F.); [email protected] (C.M.); [email protected] (S.C.); [email protected] (R.C.) 2 Department of Earth and Environmental Science, University of Pavia, Via Ferrata 9, 27100 Pavia, Italy; [email protected] (E.C.); [email protected] (G.B.) * Correspondence: [email protected]; Tel.: +39-02-6448-2700 Received: 12 August 2019; Accepted: 23 September 2019; Published: 26 September 2019 Abstract: The introduction of invasive alien plant species (IAPS) can modify plant-soil feedback, resulting in an alteration of the abiotic and biotic characteristics of ecosystems. Prunus serotina, Quercus rubra and Robinia pseudoacacia are IAPS of European temperate forests, where they can become dominant and suppress the native biodiversity. Assuming that the establishment of these invasive species may alter native forest ecosystems, this study comparatively assessed their impact on ecosystems. This study further investigated plant communities in 12 forest stands, dominated by the three IAPS and native trees, Quercus robur and Carpinus betulus (three plots per forest type), in Northern Italy, and collected soil samples. The relationships between the invasion of the three IAPS and modifications of humus forms, soil chemical properties, soil biological quality, bacterial activity and plant community structure and diversity (α-, β-, and γ-diversity) were assessed using one-way ANOVA and redundancy analyses (RDA). -

Introductory Grass Identification Workshop University of Houston Coastal Center 23 September 2017
Broadleaf Woodoats (Chasmanthium latifolia) Introductory Grass Identification Workshop University of Houston Coastal Center 23 September 2017 1 Introduction This 5 hour workshop is an introduction to the identification of grasses using hands- on dissection of diverse species found within the Texas middle Gulf Coast region (although most have a distribution well into the state and beyond). By the allotted time period the student should have acquired enough knowledge to identify most grass species in Texas to at least the genus level. For the sake of brevity grass physiology and reproduction will not be discussed. Materials provided: Dried specimens of grass species for each student to dissect Jewelry loupe 30x pocket glass magnifier Battery-powered, flexible USB light Dissecting tweezer and needle Rigid white paper background Handout: - Grass Plant Morphology - Types of Grass Inflorescences - Taxonomic description and habitat of each dissected species. - Key to all grass species of Texas - References - Glossary Itinerary (subject to change) 0900: Introduction and house keeping 0905: Structure of the course 0910: Identification and use of grass dissection tools 0915- 1145: Basic structure of the grass Identification terms Dissection of grass samples 1145 – 1230: Lunch 1230 - 1345: Field trip of area and collection by each student of one fresh grass species to identify back in the classroom. 1345 - 1400: Conclusion and discussion 2 Grass Structure spikelet pedicel inflorescence rachis culm collar internode ------ leaf blade leaf sheath node crown fibrous roots 3 Grass shoot. The above ground structure of the grass. Root. The below ground portion of the main axis of the grass, without leaves, nodes or internodes, and absorbing water and nutrients from the soil. -

Ornamental Garden Plants of the Guianas Pt. 2
Surinam (Pulle, 1906). 8. Gliricidia Kunth & Endlicher Unarmed, deciduous trees and shrubs. Leaves alternate, petiolate, odd-pinnate, 1- pinnate. Inflorescence an axillary, many-flowered raceme. Flowers papilionaceous; sepals united in a cupuliform, weakly 5-toothed tube; standard petal reflexed; keel incurved, the petals united. Stamens 10; 9 united by the filaments in a tube, 1 free. Fruit dehiscent, flat, narrow; seeds numerous. 1. Gliricidia sepium (Jacquin) Kunth ex Grisebach, Abhandlungen der Akademie der Wissenschaften, Gottingen 7: 52 (1857). MADRE DE CACAO (Surinam); ACACIA DES ANTILLES (French Guiana). Tree to 9 m; branches hairy when young; poisonous. Leaves with 4-8 pairs of leaflets; leaflets elliptical, acuminate, often dark-spotted or -blotched beneath, to 7 x 3 (-4) cm. Inflorescence to 15 cm. Petals pale purplish-pink, c.1.2 cm; standard petal marked with yellow from middle to base. Fruit narrowly oblong, somewhat woody, to 15 x 1.2 cm; seeds up to 11 per fruit. Range: Mexico to South America. Grown as an ornamental in the Botanic Gardens, Georgetown, Guyana (Index Seminum, 1982) and in French Guiana (de Granville, 1985). Grown as a shade tree in Surinam (Ostendorf, 1962). In tropical America this species is often interplanted with coffee and cacao trees to shade them; it is recommended for intensified utilization as a fuelwood for the humid tropics (National Academy of Sciences, 1980; Little, 1983). 9. Pterocarpus Jacquin Unarmed, nearly evergreen trees, sometimes lianas. Leaves alternate, petiolate, odd- pinnate, 1-pinnate; leaflets alternate. Inflorescence an axillary or terminal panicle or raceme. Flowers papilionaceous; sepals united in an unequally 5-toothed tube; standard and wing petals crisped (wavy); keel petals free or nearly so. -

Medicago Sativa L.)
ACTA BIOLOGICA CRACOVIENSIA Series Botanica 53/1: 96–101, 2011 DOI: 10.2478/v10182-011-0013-4 CYTOEMBRYOLOGICAL ANALYSIS OF CAUSES FOR POOR SEED SET IN ALFALFA (MEDICAGO SATIVA L.) RAFAŁ MÓL1, DOROTA WEIGT2*, AND ZBIGNIEW BRODA2 1Department of General Botany, Adam Mickiewicz University, Umultowska 89, 61-614 Poznań, Poland 2Department of Genetics and Plant Breeding, Poznan University of Life Sciences, Wojska Polskiego 71 C, 61-625 Poznań, Poland Received February 3, 2011; revision accepted April 21, 2011 Poor seed set is a limiting factor in alfalfa breeding, as it slows the selection response. One strategy used to over- come this problem is to search for mutations of inflorescence morphology. Long-peduncle (lp), branched-raceme (br) and top-flowering (tf) inflorescence mutations increase the number of flowers per inflorescence, but they do not improve seed set per flower. Here we assessed pollen tube growth in styles of those inflorescence mutants and we observed embryo and endosperm development in seeds 1 to 16 days after pollination (DAP). The num- ber of pollen tubes penetrating the style and the ovary was similar in all tested mutants and in the reference cul- tivar Radius. At 2 DAP, fertilized ovules were 2.7–3.9 times less numerous in certain inflorescence mutants than in the short-raceme cv. Radius. Ovule degeneration progressed at 2–4 DAP in all analyzed plants. Most ovules were not properly developed in the control cultivar (62%), nor in the forms with mutated inflorescence mor- phology (69–86%). The number of seeds per pod was lowest in the tf form despite its having the highest num- ber of ovules per ovary. -
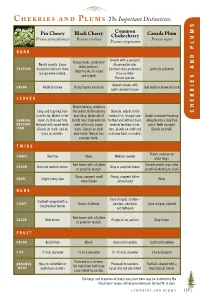
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

TREES for WESTERN NEBRASKA Justin Evertson & Bob Henrickson
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS TREES FOR WESTERN NEBRASKA Justin Evertson & Bob Henrickson. For more plant information, visit plantnebraska.org or retreenbraska.unl.edu The following species are recommended for areas in the western half of Nebraska and/or typically receive less than 20” of moisture per year. Size Range: The size range indicated for each plant is the expected average mature height x spread for Nebraska. Large Deciduous Trees (typically over 40 feet tall at maturity) NOTE ON ASH SPECIES: Native American ash trees are being decimated by Emerald Ash Borer (EAB) and the insect is now in Nebraska. NSA recommends that native ash species no longer be planted in Nebraska. 1. Ash, Manchurian - Fraxinus mandshurica (from Asia; upright growth; drought tolerant; may be resistant to EAB; 40’x 30’) 2. Catalpa, Northern - Catalpa speciosa (native; tough tree; large, heart-shaped leaves, showy flowers and long seed pods; 50’x 35’) 3. Coffeetree, Kentucky - Gymnocladus dioicus (native; amazingly adaptable; beautiful winter form; 50’x 40’) 4. Cottonwood, Eastern - Populus deltoides (majestic native; not for extremely dry sites; avoid most cultivars; 80’x 60’) 5. Cottonwood, Lanceleaf - Populus acuminata (native; naturally occurring hybrid; narrow leaves; for west. G.P.; 50’x 35’) 6. Elm, American - Ulums americana (disease resistant varieties include ‘Valley Forge’ and ‘New Harmony’; 50’x50’) 7. Elm, Japanese - Ulmus davidiana var. japonica (cold tolerant; rounded; glossy green; ‘Discovery’ is a cultivar from Manitoba Canada; 45’x 45’) 8. Elm, Rock - Ulmus thomasii (distinctive corky stems; upright habit; DED resistance in west; 50-60’x 30-40’) New Elm, Hybrids - many disease resistant hybrid elms have been developed and show promise, including: 9.