Phylogeny of Selaginellaceae: There Is Value in Morphology After All! 1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Download Document
African countries and neighbouring islands covered by the Synopsis. S T R E L I T Z I A 23 Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands by J.P. Roux Pretoria 2009 S T R E L I T Z I A This series has replaced Memoirs of the Botanical Survey of South Africa and Annals of the Kirstenbosch Botanic Gardens which SANBI inherited from its predecessor organisations. The plant genus Strelitzia occurs naturally in the eastern parts of southern Africa. It comprises three arborescent species, known as wild bananas, and two acaulescent species, known as crane flowers or bird-of-paradise flowers. The logo of the South African National Biodiversity Institute is based on the striking inflorescence of Strelitzia reginae, a native of the Eastern Cape and KwaZulu-Natal that has become a garden favourite worldwide. It sym- bolises the commitment of the Institute to champion the exploration, conservation, sustain- able use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. J.P. Roux South African National Biodiversity Institute, Compton Herbarium, Cape Town SCIENTIFIC EDITOR: Gerrit Germishuizen TECHNICAL EDITOR: Emsie du Plessis DESIGN & LAYOUT: Elizma Fouché COVER DESIGN: Elizma Fouché, incorporating Blechnum palmiforme on Gough Island PHOTOGRAPHS J.P. Roux Citing this publication ROUX, J.P. 2009. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-48-8 © Published by: South African National Biodiversity Institute. Obtainable from: SANBI Bookshop, Private Bag X101, Pretoria, 0001 South Africa. -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

Evidence-Based Medicinal Potential and Possible Role of Selaginella in the Prevention of Modern Chronic Diseases: Ethnopharmacological and Ethnobotanical Perspective
REVIEW ARTICLE Rec. Nat. Prod. X:X (2021) XX-XX Evidence-Based Medicinal Potential and Possible Role of Selaginella in the Prevention of Modern Chronic Diseases: Ethnopharmacological and Ethnobotanical Perspective Mohd Adnan 1*, Arif Jamal Siddiqui 1, Arshad Jamal 1, Walid Sabri Hamadou 1, Amir Mahgoub Awadelkareem 2, Manojkumar Sachidanandan 3 and Mitesh Patel 4 1Department of Biology, College of Science, University of Ha’il, Ha’il, P O Box 2440, Saudi Arabia 2Department of Clinical Nutrition, College of Applied Medial Sciences, University of Hail, Hail PO Box 2440, Saudi Arabia 3Department of Oral Radiology, College of Dentistry, University of Hail, Hail, PO Box 2440, Saudi Arabia 4Bapalal Vaidya Botanical Research Centre, Department of Biosciences, Veer Narmad South Gujarat University, Surat, Gujarat, India (Received November 26, 2020; Revised January 29, 2021; Accepted January 31, 2021) Abstract: Different species of the genus Selaginella are exploited for various ethnomedicinal purposes around the globe; mainly to cure fever, jaundice, hepatic disorders, cardiac diseases, cirrhosis, diarrhea, cholecystitis, sore throat, cough of lungs, promotes blood circulation, removes blood stasis and stops external bleeding after trauma and separation of the umbilical cord. Though, high content of various phytochemicals has been isolated from Selaginella species, flavonoids have been recognized as the most active component in the genus. Crude extract and different bioactive compounds of this plant have revealed various in vitro bioactivities such as, antimicrobial, antiviral, anti-diabetic, anti-mutagenic, anti-inflammatory, anti-nociceptive, anti-spasmodic, anticancer and anti-Alzheimer. However, more studies into the pharmacological activities are needed, since none of the professed bioactivity of this plant have ever been fully evaluated. -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant. -

Comparative Transcriptome Analysis Suggests Convergent Evolution Of
Alejo-Jacuinde et al. BMC Plant Biology (2020) 20:468 https://doi.org/10.1186/s12870-020-02638-3 RESEARCH ARTICLE Open Access Comparative transcriptome analysis suggests convergent evolution of desiccation tolerance in Selaginella species Gerardo Alejo-Jacuinde1,2, Sandra Isabel González-Morales3, Araceli Oropeza-Aburto1, June Simpson2 and Luis Herrera-Estrella1,4* Abstract Background: Desiccation tolerant Selaginella species evolved to survive extreme environmental conditions. Studies to determine the mechanisms involved in the acquisition of desiccation tolerance (DT) have focused on only a few Selaginella species. Due to the large diversity in morphology and the wide range of responses to desiccation within the genus, the understanding of the molecular basis of DT in Selaginella species is still limited. Results: Here we present a reference transcriptome for the desiccation tolerant species S. sellowii and the desiccation sensitive species S. denticulata. The analysis also included transcriptome data for the well-studied S. lepidophylla (desiccation tolerant), in order to identify DT mechanisms that are independent of morphological adaptations. We used a comparative approach to discriminate between DT responses and the common water loss response in Selaginella species. Predicted proteomes show strong homology, but most of the desiccation responsive genes differ between species. Despite such differences, functional analysis revealed that tolerant species with different morphologies employ similar mechanisms to survive desiccation. Significant functions involved in DT and shared by both tolerant species included induction of antioxidant systems, amino acid and secondary metabolism, whereas species-specific responses included cell wall modification and carbohydrate metabolism. Conclusions: Reference transcriptomes generated in this work represent a valuable resource to study Selaginella biology and plant evolution in relation to DT. -

Desiccation Tolerance: Phylogeny and Phylogeography
Desiccation Tolerance: Phylogeny and Phylogeography BioQUEST Workshop 2009 Resurrection Plants Desiccation tolerant Survive dehydration Survive in dormant state for extended time period Survive rehydration Xerophyta humilis, from J. Farrant web site (http://www.mcb.uct.ac.za/Staff/JMF/index.htm) Figure 2. Selaginella lepidophylla http://en.wikipedia.org/wiki/ File:Rose_of_Jericho.gif Desiccation Issues Membrane integrity Protein structure Generation of free radicals Rascia, N, La Rocca, N. 2005 Critical Reviews in Plant Sciences Solutions Repair upon hydration Prevent damage during dehydration Production/accumulation of replacement solutes Inhibition of photosynthesis http://www.cbs.dtu.dk/staff/dave/roanoke/elodeacell.jpg Evolution of Desiccation Tolerance From Oliver, et al 2000. Plant Ecology Convergent Evolution Among Vascular Plants From Oliver, et al 2000. Plant Ecology Desiccation Tolerance in Vascular Plants Seeds Pollen Spores Osmotic stress Desertification 40% of Earth’s surface 38% of population Low productivity Lack of water Depletion of soil Loss of soil Delicate system Using Evolutionary Relationships to Identify Genes for Crop Enhancement If desiccation sensitive plants have retained genes involved in desiccation tolerance, perhaps expression of those genes can be genetically modified to enhance desiccation tolerance. Expression patterns in dehydratingTortula and Xerophyta Michael Luth Xerophyta humilis, from J. Farrant web site (http://www.mcb.uct.ac.za/Staff/JMF/index.htm) ? Methods Occurrence data for Anastatica hierochuntic: o Downloaded from the Global Biodiversity Information Facility (gbif.org). o Of 127 available occurrence points, only 68 were georeferenced with latitude and longitude information. Environmental layers: Precipitation and temperature from the IPCC climate dataset Niche Modeling: Maximum Entropy (Maxent) Maxent principle is to estimate the probability distribution such as the spatial distribution of a species. -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -
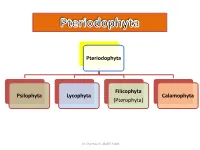
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

Topic 11: Land Plants, Part 1 (Bryophytes, Ferns & Fern Allies)
BIOL 221 – Concepts of Botany Spring 2008 Topic 11: Land Plants, part 1 (Bryophytes, Ferns & Fern Allies) A. Objectives for today’s lab . 1. Get to know 2 of the three groups of bryophytes (liverworts & mosses). 2. Get to know some of the Ferns and Fern Allies, which include some of the earliest lineages of vascular plants (represented today by Psilotum, Lycopodium, Equisetum, & Ferns—Boston (sword) fern, Staghorn fern) 3. Think about the morphological/anatomical innovations that are represented by each. Place these in the context of the origins of leaves, roots, and the fossil record and green plant phylogeny. 4. Know the general sequence of appearance: Green Algae ==> Bryophyte Lineages ==> Lycopod & Horsetail Lineages ==> Fern Lineages ==> Seed Plants (Gymnosperms & Angiosperms) B. Green Plant Phylogeny . Concepts of Botany, (page 1 of 12) C. NonVascular Free-Sporing Land Plants (Bryophytes) . C1. Liverworts . a. **LIVING MATERIAL**: Marchantia &/or Conacephalum (thallose liverworts). The conspicuous green plants are the gametophytes. With a dissecting scope, observe the polygonal outlines of the air chambers. On parts of the thallus are drier, it is easy to see the pore opening to the chamber. These are not stomata. They cannot open and close and the so the thallus can easily dry out if taking from water. Note the gemmae cups on some of that thalli. Ask your instructor what these are for. DRAW THEM TOO. b. **SLIDES**. Using the compound microscope, make observations of the vegetative and reproductive parts of various liverwort species. Note the structure of the air-chambers and the photosynthetic cells inside on the Marchantia sections! Concepts of Botany, (page 2 of 12) C2. -

Plastid Genomes of the Early Vascular Plant Genus Selaginella Have Unusual Direct Repeat Structures and Drastically Reduced Gene Numbers
International Journal of Molecular Sciences Article Plastid Genomes of the Early Vascular Plant Genus Selaginella Have Unusual Direct Repeat Structures and Drastically Reduced Gene Numbers Hyeonah Shim 1, Hyeon Ju Lee 1, Junki Lee 1,2, Hyun-Oh Lee 1,2, Jong-Hwa Kim 3, Tae-Jin Yang 1,* and Nam-Soo Kim 4,* 1 Department of Agriculture, Forestry and Bioresources, Plant Genomics & Breeding Institute, Research Institute of Agriculture and Life Sciences, College of Agriculture & Life Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea; [email protected] (H.S.); [email protected] (H.J.L.); [email protected] (J.L.); [email protected] (H.-O.L.) 2 Phyzen Genomics Institute, Seongnam 13558, Korea 3 Department of Horticulture, Kangwon National University, Chuncheon 24341, Korea; [email protected] 4 Department of Molecular Bioscience, Kangwon National University, Chuncheon 24341, Korea * Correspondence: [email protected] (T.-J.Y.); [email protected] (N.-S.K.); Tel.: +82-2-880-4547 (T.-J.Y.); +82-33-250-6472 (N.-S.K.) Abstract: The early vascular plants in the genus Selaginella, which is the sole genus of the Selaginel- laceae family, have an important place in evolutionary history, along with ferns, as such plants are valuable resources for deciphering plant evolution. In this study, we sequenced and assembled the plastid genome (plastome) sequences of two Selaginella tamariscina individuals, as well as Se- laginella stauntoniana and Selaginella involvens. Unlike the inverted repeat (IR) structures typically found in plant plastomes, Selaginella species had direct repeat (DR) structures, which were confirmed by Oxford Nanopore long-read sequence assembly.