Carbon Discovered: Known Since Ancient Times. It Was First Recognized
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
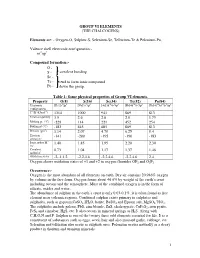
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

This Ubiquitous Carbon…
Engineering Physics Department Presents Dr. Cristian Contescu Senior Research Staff, Materials Science and Technology Division Oak Ridge National Laboratory This ubiquitous carbon… Abstract: After Stone Age, Bronze Age, and Iron Age, and after the Silicon Age of the informational revolution, the technologies of 21st century are marked by the ubiquitous presence of various forms of carbon allotropes. For a very long time, diamond and graphite were the only known carbon allotropes, but that has changed with the serendipitous discovery of fullerenes, carbon nanotubes, and graphene. Every ten or fifteen years scientists unveil new forms of carbons with new and perplexing properties, while computations suggest that the carbon’s family still has members unknown to us today. At a dramatically accelerated pace, new carbon forms find their place at the leading edge of scientific and technological innovations. At the same time traditional forms of carbon are being used in new and exciting applications that make our life safer, healthier, and more enjoyable. The 21st century may soon be recognized as the Age of Carbon forms. This educational talk will show how carbon, the fourth most abundant element in the Galaxy and the basis of life on Earth, was the engine of most important technological developments throughout the history of civilization. It will emphasize the ability of carbon atoms to generate a variety of mutual combinations and with many other chemical elements. These properties have placed carbon at the core of numerous inventions that define our civilization, while emerging new technologies open a rich path for value-added products in today’s market. -

Amarsinha D. Nikam Vital Force Is Oxygen Extrait Du Livre Vital Force Is Oxygen De Amarsinha D
Amarsinha D. Nikam Vital Force is Oxygen Extrait du livre Vital Force is Oxygen de Amarsinha D. Nikam Éditeur : B. Jain http://www.editions-narayana.fr/b9138 Sur notre librairie en ligne vous trouverez un grand choix de livres d'homéopathie en français, anglais et allemand. Reproduction des extraits strictement interdite. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern, Allemagne Tel. +33 9 7044 6488 Email [email protected] http://www.editions-narayana.fr Oxygen Oxygen is derived from the Greek word, where oxys means acid, literally sharp from the taste of acids and genes means producer, literally begetter. It is the element with atomic number 8 and represented by the symbol 'O'. It is a highly reactive non-metallic period 2 element that readily forms compounds (notably oxides) with almost all other elements. Oxygen is the third most abundant element in the universe by mass after hydrogen and helium. Diatomic oxygen gas constitutes 21% of the volume of air. Water is the most familiar oxygen compound. OXYGEN HISTORY Oxygen makes up 21% of the atmosphere we breathe, but it was not discovered as a separate gas until the late i8th century. Oxygen was independently discovered by Carl Wilhelm Scheele, in Uppsala in 1773 or earlier and by Joseph Priestley in Wiltshire, in 1774. The name oxygen was coined in 1777 by Antoine Lavoisier. Although oxygen plays a life-supporting role, it took Narayana Verlag, 79400 Kandern Tel.: 0049 7626 974 970 0 Excerpt from Dr. Amarsinha D.Nikam: Vital Force is Oxygen about 150 years for the gas to be used in a proper manner for patients. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Sp Carbon Chain Interaction with Silver Nanoparticles Probed by Surface Enhanced Raman Scattering
sp carbon chain interaction with silver nanoparticles probed by Surface Enhanced Raman Scattering A. Lucotti1, C. S. Casari2, M. Tommasini1, A. Li Bassi2, D. Fazzi1, V. Russo2, M. Del Zoppo1, C. Castiglioni1, F. Cataldo3, C. E. Bottani2, G. Zerbi1 1 Dipartimento di Chimica, Materiali e Ingegneria Chimica ‘G. Natta’ and NEMAS - Center for NanoEngineered MAterials and Surfaces, Politecnico di Milano, Piazza Leonardo da Vinci 32, I-20133 Milano, Italy 2 Dipartimento di Energia and NEMAS - Center for NanoEngineered MAterials and Surfaces, Politecnico di Milano, via Ponzio 34/3, I-20133 Milano, Italy 3 Actinium Chemical Research srl, via Casilina 1626/A, 00133 Roma, Italy and INAF – Osservatorio Astrofisico di Catania, Via S. Sofia 78, 95123 Catania, Italy Abstract Surface Enhanced Raman Spectroscopy (SERS) is exploited here to investigate the interaction of isolated sp carbon chains (polyynes) in a methanol solution with silver nanoparticles. Hydrogen-terminated polyynes show a strong interaction with silver colloids used as the SERS active medium revealing a chemical SERS effect. SERS spectra after mixing polyynes with silver colloids show a noticeable time evolution. Experimental results, supported by density functional theory (DFT) calculations of the Raman modes, allow us to investigate the behaviour and stability of polyynes of different lengths and the overall sp conversion towards sp2 phase. 1 2 1. Introduction Linear carbon chains with sp hybridization represent one of the simplest one dimensional systems and have therefore attracted a great interest for many years [1, 2]. sp chains can display two types of carbon-carbon bonding: polyynes, chains with single-triple alternating bonds (…-C≡C- C≡C-…) and polycumulenes, chains with all double bonds (…=C=C=C=…). -

Agricultural Soil Carbon Credits: Making Sense of Protocols for Carbon Sequestration and Net Greenhouse Gas Removals
Agricultural Soil Carbon Credits: Making sense of protocols for carbon sequestration and net greenhouse gas removals NATURAL CLIMATE SOLUTIONS About this report This synthesis is for federal and state We contacted each carbon registry and policymakers looking to shape public marketplace to ensure that details investments in climate mitigation presented in this report and through agricultural soil carbon credits, accompanying appendix are accurate. protocol developers, project developers This report does not address carbon and aggregators, buyers of credits and accounting outside of published others interested in learning about the protocols meant to generate verified landscape of soil carbon and net carbon credits. greenhouse gas measurement, reporting While not a focus of the report, we and verification protocols. We use the remain concerned that any end-use of term MRV broadly to encompass the carbon credits as an offset, without range of quantification activities, robust local pollution regulations, will structural considerations and perpetuate the historic and ongoing requirements intended to ensure the negative impacts of carbon trading on integrity of quantified credits. disadvantaged communities and Black, This report is based on careful review Indigenous and other communities of and synthesis of publicly available soil color. Carbon markets have enormous organic carbon MRV protocols published potential to incentivize and reward by nonprofit carbon registries and by climate progress, but markets must be private carbon crediting marketplaces. paired with a strong regulatory backing. Acknowledgements This report was supported through a gift Conservation Cropping Protocol; Miguel to Environmental Defense Fund from the Taboada who provided feedback on the High Meadows Foundation for post- FAO GSOC protocol; Radhika Moolgavkar doctoral fellowships and through the at Nori; Robin Rather, Jim Blackburn, Bezos Earth Fund. -

Environment and Ecology BHM 403 Techno India
Environment and Ecology BHM 403 Techno India ENVIRONMENT AND ECOLOGY BHM 403 (2008 -2017) PREPARED BY ANIS CHATTOPADHYAY ASST. PROFESSOR TECHNO INDIA EM-4/1, SECTOR –V, SALT LAKE KOLKATA -700091 1 Environment and Ecology BHM 403 Techno India 2008 GROUP – A ( Multiple Choice Type Questions ) 1. Choose correct answer from the given alternatives in each of the following questions : 10x1 = 10 i) Environmental Studies involve studies of a) evolution of life b) all aspects of human environment c) nitrogen cycle d) oxygen cycle. b ii) "Itai itai' disease is caused by a) Zinc b) Cadmium c) Mercury d) Iron. b iii) El Nino starts from a) Mediterranean coast b) Chinese coast c) South American cost d) Indian cost. C iv) The Greenhouse effect is due to a) Carbon dioxide, water vapour, methane and chlorofluorocarbons b) Nitrogen oxide c) Sulphur oxide d) Carbon monoxide. A v) The Ganga pollution is due to dumping of a) domestic and industrial sewages b) waste from forest c) food waste d) hospital waste. A vi) Biotic factor of ecosystem is a) Solar energy b) Temperature c) Soil d) Plants and animals. D vii) Medha Patkar is involved in a) Chipko movement b) Silent Valley movement c) Narmada Bachao movement d) none of these. C viii) The "Kyoto Protocol" is related with a) air pollution b) noise pollution c) water pollution d) none of these. A ix) BOD stands as a) Biochemical Oxygen Demand b) Biological Oxygen Demand c) Biggest Oxygen Demand d) Blown Out Dose. B x) The protective shield for life on earth is a) Carbon dioxide b) Ozone c) Oxygen d) Hydrogen. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

Glossary of Terms for Carbon Dioxide Capture and Storage
CCS Defined A glossary of terms for Carbon dioxide Capture and Storage Deliverable D.73 of the STEMM-CCS project - 2016 INTRODUCTION: This glossary – ‘CCS Defined’ - has been brought together from many sources, and following comments and advice from co-workers on the Strategies for Environmental Monitoring of Marine Carbon Capture and Storage, STEMM- CCS (654462), CO2 Capture from Cement Production, CEMCAP (641185) and Low Emissions Intensity Lime and Cement, LEILAC (654465) Projects, which form a group under the EC H2020 Carbon Capture and Storage Programme. ‘CCS defined’ (deliverable D.73) comprises an update and broadening of an early glossary (Boot et al, 2013. The Language of CCS) to bring together a comprehensive set of definitions concerned with sub-seabed carbon dioxide capture and storage (CCS) produced as a deliverable of the FP7, ECO2 project (http://www.eco2-project.eu/). The ECO2 “Language of CCS’ was concerned primarily with aspects of sub-seabed storage, ‘CCS Defined’ includes other topics reflecting additional language required by the LEILAC and CEMCAP projects, especially elements of capture technologies, and is widened to include storage in general. It is, therefore, a more complete glossary which should prove useful beyond the immediate projects for which it is written. The aim of producing ‘CCS Defined’ is in the first instance to provide a common vocabulary intended to minimise misunderstandings and confusion across the various scientific disciplines working within CCS. It is NOT intended to provide a document with any legal standing, whatsoever. As with the previous publication, ‘CCS Defined’ has drawn upon a wide range of sources within the relevant literature and across numerous websites so this glossary is very much a compilation of many ideas.