Carbon Allotropes and Their Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allan Hugh Macdonald Date and Place of Birth
10/31/2014 CURRICULUM VITAE ALLAN H. MACDONALD Full name: Allan Hugh MacDonald Date and place of birth: December 1, 1951 Antigonish, Nova Scotia, Canada Citizenships: Canadian and American Present address: 2519 Harris Boulevard Austin, Texas 78703 USA Phone (512) 495-9192 Institutional affiliation: The University of Texas at Austin Austin, Texas 78712 Phone (512) 232-9113 FAX (512) 471-9621 e-mail: [email protected] Title: Sid W. Richardson Foundation Regents Chair Field of Specialization: Condensed Matter Theory Employment: September 1973 -- April 1978 Ph.D. Student University of Toronto May 1978 -- October 1980 Research Associate --- National Research Council November 1980 -- June 1982 Assistant Research Officer -- National Research Council of Canada August 1982 -- August 1987 Associate Research Officer --- National Research Council of Canada September 1987 -- August 1992 Professor of Physics --- Indiana University September 1992 -- August 2000 Distinguished Professor of Physics --- Indiana University September 2000 -- present Sid W. Richardson Foundation Regents Chair --- The University of Texas at Austin 1 10/31/2014 Scholarships and Honors: President's Scholarship, St. Francis Xavier University, 1969–1973 Governor-General's Medal, St. Francis Xavier University, 1973 (Highest academic standing in graduating class) NSERC 1967 Science Scholarship, University of Toronto, 1973–1977 Herzberg Medal, 1987 (Awarded by the Canadian Association of Physicists) Fellow of the American Physical Society, 1989 Sid W. Richardson Foundation Regents -
Group Vi Elements (The Chalcogens)
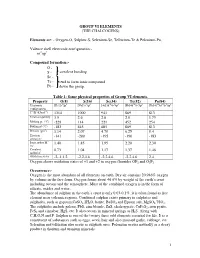
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Moiré Band Topology in Twisted Bilayer Graphene
Moiré Band Topology in Twisted Bilayer Graphene Chao Ma, † Qiyue Wang, ‡ Scott Mills,§ Xiaolong Chen, †# Bingchen Deng, † Shaofan Yuan, † Cheng Li, † Kenji Watanabe, || Takashi Taniguchi, || Du Xu, *§ Fan Zhang, *‡ and Fengnian Xia*† †Department of Electrical Engineering, Yale University, New Haven, Connecticut 06511, USA ‡Department of Physics, The University of Texas at Dallas, Richardson, TX 7508, USA §Department of Physics and Astronomy, Stony Brook University, Stony Brook, NY11794, USA ||National Institute for Materials Science, 1-1 Namiki, Tsukuba 305-0044, Japan 1 ABSTRACT Recently twisted bilayer graphene (t-BLG) emerges as a new strongly correlated physical platform near a magic twist angle, which hosts many exciting phenomena such as the Mott-like insulating phases, unconventional superconducting behavior and emergent ferromagnetism. Besides the apparent significance of band flatness, band topology may be another critical element in determining strongly correlated twistronics yet receives much less attention. Here we report compelling evidence for nontrivial noninteracting band topology of t-BLG moiré Dirac bands through a systematic nonlocal transport study, in conjunction with an examination rooted in K- theory. The moiré band topology of t-BLG manifests itself as two pronounced nonlocal responses in the electron and hole superlattice gaps. We further show that the nonlocal responses are robust to the interlayer electric field, twist angle, and edge termination, exhibiting a universal scaling law. While an unusual symmetry of t-BLG trivializes Berry curvature, we elucidate that two Z2 invariants characterize the topology of the moiré Dirac bands, validating the topological edge origin of the observed nonlocal responses. Our findings not only provide a new perspective for understanding the emerging strongly correlated phenomena in twisted van der Waals heterostructures, but also suggest a potential strategy to achieve topologically nontrivial metamaterials from topologically trivial quantum materials based on twist engineering. -

Exploring the Electrical Properties of Twisted Bilayer Graphene
Linfield University DigitalCommons@Linfield Senior Theses Student Scholarship & Creative Works 5-2019 Exploring the Electrical Properties of Twisted Bilayer Graphene William Shannon Linfield College Follow this and additional works at: https://digitalcommons.linfield.edu/physstud_theses Part of the Condensed Matter Physics Commons, Energy Systems Commons, Engineering Physics Commons, Materials Science and Engineering Commons, and the Power and Energy Commons Recommended Citation Shannon, William, "Exploring the Electrical Properties of Twisted Bilayer Graphene" (2019). Senior Theses. 45. https://digitalcommons.linfield.edu/physstud_theses/45 This Thesis (Open Access) is protected by copyright and/or related rights. It is brought to you for free via open access, courtesy of DigitalCommons@Linfield, with permission from the rights-holder(s). Your use of this Thesis (Open Access) must comply with the Terms of Use for material posted in DigitalCommons@Linfield, or with other stated terms (such as a Creative Commons license) indicated in the record and/or on the work itself. For more information, or if you have questions about permitted uses, please contact [email protected]. Exploring the Electrical Properties of Twisted Bilayer Graphene William Shannon A THESIS Presented to the Department of Physics LINFIELD COLLEGE McMinnville, Oregon In partial fulfillment of the requirements for the Degree of BACHELOR OF SCIENCE May, 2019 THESIS COPYRIGHT PERMISSIONS Pleaseread this document carefully before signing. If you have questions about any of these permissions,please contact the DigitalCommonsCoordinator. Title of the Thesis: Exploring the Electrical Properties of Twisted Bilayer Graphene Author's Name: (Last name, first name) Shannon, William111 Advisor's Name DigitalCommons@Linfield(DC@L) is our web-based, open access-compliantinstitutional repository for digital content produced by Linfield faculty, students, staff, and their collaborators. -

Critical Point for Bose–Einstein Condensation of Excitons in Graphite
Critical point for Bose–Einstein condensation of excitons in graphite Jinhua Wanga,b , Pan Niea,b , Xiaokang Lia,b, Huakun Zuoa,b, Benoˆıt Fauque´ c, Zengwei Zhua,b,1 , and Kamran Behniad aWuhan National High Magnetic Field Center, Huazhong University of Science and Technology, Wuhan 430074, China; bSchool of Physics, Huazhong University of Science and Technology, Wuhan 430074, China; cJeunes Equipes´ de l’Institut de Physique, Unite´ Mixte de Service et de Recherche 3573, CNRS, College` de France, Paris Sciences et Lettres Research University, 75231 Paris Cedex 05, France; and dLaboratoire de Physique et d’Etude´ des Materiaux,´ CNRS, Ecole´ Superieure´ de Physique et de Chimie Industrielles Paris, Paris Sciences et Lettres Research University, 75005 Paris, France Edited by Zachary Fisk, University of California, Irvine, CA, and approved October 15, 2020 (received for review June 22, 2020) An exciton is an electron–hole pair bound by attractive Coulomb nature of EI unexpected in the alternative Peierls-driven charge interaction. Short-lived excitons have been detected by a vari- density wave (CDW) (9). Other indirect signatures of BEC ety of experimental probes in numerous contexts. An excitonic transition have been reported in two-dimensional systems, such insulator, a collective state of such excitons, has been more elu- as quantum wells (11), graphene (12–14), and transition metal sive. Here, thanks to Nernst measurements in pulsed magnetic dichalcogenides heterostructures (15, 16). fields, we show that in graphite there is a critical temperature Here, we present the case of graphite subject to strong mag- (T = 9.2 K) and a critical magnetic field (B = 47 T) for Bose–Einstein netic field where the existence of a thermodynamic phase transi- condensation of excitons. -

The Era of Carbon Allotropes Andreas Hirsch
commentary The era of carbon allotropes Andreas Hirsch Twenty-five years on from the discovery of 60C , the outstanding properties and potential applications of the synthetic carbon allotropes — fullerenes, nanotubes and graphene — overwhelmingly illustrate their unique scientific and technological importance. arbon is the element in the periodic consist of extended networks of sp3- and 1985, with the advent of fullerenes (Fig. 1), table that provides the basis for life sp2 -hybridized carbon atoms, respectively. which were observed for the first time by Con Earth. It is also important for Both forms show unique physical properties Kroto et al.3. This serendipitous discovery many technological applications, ranging such as hardness, thermal conductivity, marked the beginning of an era of synthetic from drugs to synthetic materials. This lubrication behaviour or electrical carbon allotropes. Now, as we celebrate role is a consequence of carbon’s ability conductivity. Conceptually, many other buckminsterfullerene’s 25th birthday, it is to bind to itself and to nearly all elements ways to construct carbon allotropes are also the time to reflect on a growing family in almost limitless variety. The resulting possible by altering the periodic binding of synthetic carbon allotropes, which structural diversity of organic compounds motif in networks consisting of sp3-, sp2- includes the synthesis of carbon nanotubes and molecules is accompanied by a broad and sp-hybridized carbon atoms1,2. As a in 19914 and the rediscovery of graphene range of -

Wafer Scale Homogeneous Bilayer Graphene Films by Chemical Vapor
Wafer Scale Homogeneous Bilayer Graphene Films by Chemical Vapor Deposition Seunghyun Lee§, Kyunghoon Lee§, Zhaohui Zhong * Department of Electrical Engineering and Computer Science, University of Michigan Ann Arbor, MI 48109, USA § These authors contributed equally to this work. *Corresponding author. Electronic mail: [email protected] ABSTRACT The discovery of electric field induced bandgap opening in bilayer graphene opens new door for making semiconducting graphene without aggressive size scaling or using expensive substrates. However, bilayer graphene samples have been limited to µm2 size scale thus far, and synthesis of wafer scale bilayer graphene posts tremendous challenge. Here we report homogeneous bilayer graphene films over at least 2 inch × 2 inch area, synthesized by chemical vapor deposition on copper foil and subsequently transferred to arbitrary substrates. The bilayer nature of graphene film is verified by Raman spectroscopy, atomic force microscopy (AFM), and transmission electron microscopy (TEM). Importantly, spatially resolved Raman spectroscopy confirms a bilayer coverage of over 99%. The homogeneity of the film is further supported by electrical transport measurements on dual-gate bilayer graphene transistors, in which bandgap opening is observed in 98% of the devices. 1 KEYWORDS Graphene, bilayer, chemical vapor deposition, wafer scale, bandgap opening Single and few-layer graphene1-5 are promising materials for post-silicon electronics because of their potential of integrating bottom-up nanomaterial synthesis with top-down lithographic fabrication at wafer scale.4,6 However, single layer graphene is intrinsically semimetal; introducing energy bandgap requires patterning nanometer-width graphene ribbons7-9 or utilizing special substrates.10-12 Bilayer graphene, instead, has an electric field induced bandgap up to 250 meV,13-18 thus eliminating the need for extreme scaling or costly substrates. -

The Raman Spectrum of Amorphous Diamond
" r / J 1 U/Y/ — f> _ - ^ /lj / AU9715866 THE RAMAN SPECTRUM OF AMORPHOUS DIAMOND S.Prawer, K.W. Nugent, D.N. Jamieson School of Physics and Microanalytical Research Centre University of Melbourne, Parkville, Victoria, Australia, 3052. ABSTRACT: We present the Raman spectrum of an amorphous, fully sp^-bonded carbon network. The reduced Raman spectrum agrees closely with the calculated density of states of diamond. The results have been obtained from nanoclusters produced deep inside a single crystal diamond irradiated with MeV He ions. The deep implantation creates amorphous sp3 bonded C clusters along the ion tracks, within a largely intact diamond matrix. The matrix maintains the clusters under high pressure, preventing the relaxation to sp% bonded structures. Sharp peaks associated with defect structures unique to MeV ion implantation are observed at 1422, 1447, 1467, 1496, 1540, 1563, 1631, 1649, 1683 and 1726 cm'1. We also observe a shoulder in the reduced Raman spectrum at about 1120 cm'1 which we tentatively attribute to quantum confinement effects in the carbon nanoclusters. The results provide the Raman signature that might be expected from tetrahedrally bonded amorphous carbon films with no graphite-like amorphous components. 2 Tetrahedrally bonded amorphous (‘diamond-like ’) carbon has attracted a great deal of both experimental and theoretical interest of the past few years [1]. There have been numerous efforts to model the vibrational spectrum of sp3 bonded amorphous carbon networks, but until now there has been no experimental confirmation, in the form of a Raman spectrum, to test the accuracy of these model calculations. This is primarily because most sp3 rich amorphous carbon films contain a minimum of 5-15% of sp2 bonded carbon. -

Closed Network Growth of Fullerenes
ARTICLE Received 9 Jan 2012 | Accepted 18 Apr 2012 | Published 22 May 2012 DOI: 10.1038/ncomms1853 Closed network growth of fullerenes Paul W. Dunk1, Nathan K. Kaiser2, Christopher L. Hendrickson1,2, John P. Quinn2, Christopher P. Ewels3, Yusuke Nakanishi4, Yuki Sasaki4, Hisanori Shinohara4, Alan G. Marshall1,2 & Harold W. Kroto1 Tremendous advances in nanoscience have been made since the discovery of the fullerenes; however, the formation of these carbon-caged nanomaterials still remains a mystery. Here we reveal that fullerenes self-assemble through a closed network growth mechanism by incorporation of atomic carbon and C2. The growth processes have been elucidated through experiments that probe direct growth of fullerenes upon exposure to carbon vapour, analysed by state-of-the-art Fourier transform ion cyclotron resonance mass spectrometry. Our results shed new light on the fundamental processes that govern self-assembly of carbon networks, and the processes that we reveal in this study of fullerene growth are likely be involved in the formation of other carbon nanostructures from carbon vapour, such as nanotubes and graphene. Further, the results should be of importance for illuminating astrophysical processes near carbon stars or supernovae that result in C60 formation throughout the Universe. 1 Department of Chemistry and Biochemistry, Florida State University, 95 Chieftan Way, Tallahassee, Florida 32306, USA. 2 Ion Cyclotron Resonance Program, National High Magnetic Field Laboratory, Florida State University, 1800 East Paul Dirac Drive, Tallahassee, Florida 32310, USA. 3 Institut des Matériaux Jean Rouxel, CNRS UMR 6502, Université de Nantes, BP32229 Nantes, France. 4 Department of Chemistry and Institute for Advanced Research, Nagoya University, Nagoya 464-8602, Japan. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Evidence for Glass Behavior in Amorphous Carbon
Journal of C Carbon Research Article Evidence for Glass Behavior in Amorphous Carbon Steven Best , Jake B. Wasley, Carla de Tomas, Alireza Aghajamali , Irene Suarez-Martinez * and Nigel A. Marks * Department of Physics and Astronomy, Curtin University, Perth, WA 6102, Australia; [email protected] (S.B.); [email protected] (J.B.W.); [email protected] (C.d.T.); [email protected] (A.A.) * Correspondence: [email protected] (I.S.-M.); [email protected] (N.A.M.) Received: 15 July 2020; Accepted: 24 July 2020; Published: 30 July 2020 Abstract: Amorphous carbons are disordered carbons with densities of circa 1.9–3.1 g/cc and a mixture of sp2 and sp3 hybridization. Using molecular dynamics simulations, we simulate diffusion in amorphous carbons at different densities and temperatures to investigate the transition between amorphous carbon and the liquid state. Arrhenius plots of the self-diffusion coefficient clearly demonstrate that there is a glass transition rather than a melting point. We consider five common carbon potentials (Tersoff, REBO-II, AIREBO, ReaxFF and EDIP) and all exhibit a glass transition. Although the glass-transition temperature (Tg) is not significantly affected by density, the choice of potential can vary Tg by up to 40%. Our results suggest that amorphous carbon should be interpreted as a glass rather than a solid. Keywords: amorphous carbon; liquid carbon; glass-transition temperature; molecular dynamics 1. Introduction Amorphous carbons are often described as one of the allotropes of carbon, along with graphite, diamond and fullerenes. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1.