Basic Keyword List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
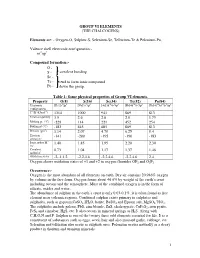
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Photochemistry of Highly Excited States R
COMMENTARY COMMENTARY Photochemistry of highly excited states R. D. Levinea,b,c,1 These days on earth we are photochemically reasonably A forceful illustration of the benefits of the synergy of well shielded. The far UV radiation is filtered by the ozone experiment and theory in understanding the detailed and other components of the atmosphere. In the iono- electronic and nuclear dynamics in highly excited states is sphere and beyond there is much challenging chemistry provided by the very recent work reported by Peters et al. induced by such higher-energy photons. The shielding by (8). Methyl azide is pumped by an 8-eV vacuum UV (VUV) the atmosphere also makes demands on such experi- pulse of 10-fs duration and probed by a second 1.6-eV in- ments in the laboratory. There is, however, recent interest frared (IR) pulse that ionizes the molecule. The experiment largely driven by technology that makes higher resolution as seen through the eyes of a theorist is shown in Fig. 1. The possible. The experimental technology is advanced light pump pulse prepares a single excited electronic state, la- sources that offer far higher intensities, sharper wave- beled as S8, that theory shows has a mixed valence and length definition or, complementarily, short-duration Rydberg character. It is unusual that a single excited state pulses. The two alternatives make a whole because of is accessed at such a high energy. The high-level computa- the quantum mechanical time–energy uncertainty princi- tions reported by Peters et al. are quite clear that the ple that makes short pulses necessarily broad in energy. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

Laboratory Astrophysics: from Observations to Interpretation
April 14th – 19th 2019 Jesus College Cambridge UK IAU Symposium 350 Laboratory Astrophysics: From Observations to Interpretation Poster design by: D. Benoit, A. Dawes, E. Sciamma-O’Brien & H. Fraser Scientific Organizing Committee: Local Organizing Committee: Farid Salama (Chair) ★ P. Barklem ★ H. Fraser ★ T. Henning H. Fraser (Chair) ★ D. Benoit ★ R Coster ★ A. Dawes ★ S. Gärtner ★ C. Joblin ★ S. Kwok ★ H. Linnartz ★ L. Mashonkina ★ T. Millar ★ D. Heard ★ S. Ioppolo ★ N. Mason ★ A. Meijer★ P. Rimmer ★ ★ O. Shalabiea★ G. Vidali ★ F. Wa n g ★ G. Del-Zanna E. Sciamma-O’Brien ★ F. Salama ★ C. Wa lsh ★ G. Del-Zanna For more information and to contact us: www.astrochemistry.org.uk/IAU_S350 [email protected] @iaus350labastro 2 Abstract Book Scheduley Sunday 14th April . Pg. 2 Monday 15th April . Pg. 3 Tuesday 16th April . Pg. 4 Wednesday 17th April . Pg. 5 Thursday 18th April . Pg. 6 Friday 19th April . Pg. 7 List of Posters . .Pg. 8 Abstracts of Talks . .Pg. 12 Abstracts of Posters . Pg. 83 yPlenary talks (40') are indicated with `P', review talks (30') with `R', and invited talks (15') with `I'. Schedule Sunday 14th April 14:00 - 17:00 REGISTRATION 18:00 - 19:00 WELCOME RECEPTION 19:30 DINNER BAR OPEN UNTIL 23:00 Back to Table of Contents 2 Monday 15th April 09:00 { 10:00 REGISTRATION 09:00 WELCOME by F. Salama (Chair of SOC) SESSION 1 CHAIR: F. Salama 09:15 E. van Dishoeck (P) Laboratory astrophysics: key to understanding the Universe From Diffuse Clouds to Protostars: Outstanding Questions about the Evolution of 10:00 A. -
Infrared and UV-Visible Time-Resolved Techniques for the Study of Tetrapyrrole-Based Proteins
Infrared and UV-visible time-resolved techniques for the study of tetrapyrrole-based proteins A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy (PhD) in the Faculty of Life Sciences 2013 Henry John Russell Contents List of Tables .......................................................................................................................................................................... 5 List of Figures ........................................................................................................................................................................ 6 Abstract ...................................................................................................................................................................................10 Declaration ............................................................................................................................................................................11 Copyright Statement ........................................................................................................................................................11 Acknowledgements ..........................................................................................................................................................12 Abbreviations and Symbols ..........................................................................................................................................13 Preface and Thesis Structure .......................................................................................................................................16 -

2. Allotropy in Carbon the Property Due to Which an Element Exists In
SUBJECT-CHEMISTRY DATE- 22/11/2020 CLASS- X TOPICS- Chap. 4 Carbon and its Compounds Carbon is one of the most essential components of living organisms. There are two stable isotopes of carbon C-12 and C-13. After these two one more isotope of carbon is present C-14. Carbon is used for radiocarbon dating One of the most amazing properties of carbon is its ability to make long carbon chains and rings. This property of carbon is known as catenation. Carbon has many special abilities out of all one unique ability is that carbon forms double or triple bonds with itself and with other electronegative atoms like oxygen and nitrogen. These two properties of carbon i.e catenation and multiple bond formation, it has the number of allotropic forms. Allotrope is nothing but the existence of an element in many forms which will have different physical property but will have similar chemical properties and its forms are called allotropes of allotropic forms. Allotropes are defined as the two or more physical forms of one element. These allotropes are all based on carbon atoms but exhibit different physical properties, especially with regard to hardness. The two common, crystalline allotropes of carbon are diamond and graphite. Carbon shows allotropy because it exists in different forms of carbon. Though these allotropes of carbon have a different crystal structure and different physical properties, their chemical properties are the same and show similar chemical properties. Both diamond and graphite have symbol C. Both give off carbon dioxide when strongly heated in the presence of oxygen. -

Angwcheminted, 2000, 39, 2587-2631.Pdf
REVIEWS Femtochemistry: Atomic-Scale Dynamics of the Chemical Bond Using Ultrafast Lasers (Nobel Lecture)** Ahmed H. Zewail* Over many millennia, humankind has biological changes. For molecular dy- condensed phases, as well as in bio- thought to explore phenomena on an namics, achieving this atomic-scale res- logical systems such as proteins and ever shorter time scale. In this race olution using ultrafast lasers as strobes DNA structures. It also offers new against time, femtosecond resolution is a triumph, just as X-ray and electron possibilities for the control of reactivity (1fs 10À15 s) is the ultimate achieve- diffraction, and, more recently, STM and for structural femtochemistry and ment for studies of the fundamental and NMR spectroscopy, provided that femtobiology. This anthology gives an dynamics of the chemical bond. Ob- resolution for static molecular struc- overview of the development of the servation of the very act that brings tures. On the femtosecond time scale, field from a personal perspective, en- about chemistryÐthe making and matter wave packets (particle-type) compassing our research at Caltech breaking of bonds on their actual time can be created and their coherent and focusing on the evolution of tech- and length scalesÐis the wellspring of evolution as a single-molecule trajec- niques, concepts, and new discoveries. the field of femtochemistry, which is tory can be observed. The field began the study of molecular motions in the with simple systems of a few atoms and Keywords: femtobiology ´ femto- hitherto unobserved ephemeral transi- has reached the realm of the very chemistry ´ Nobel lecture ´ physical tion states of physical, chemical, and complex in isolated, mesoscopic, and chemistry ´ transition states 1. -

Femtochemistry' Ahmed Zewail Wins the 1999 Nobel Prize in Chemistry
GENERAL I ARTICLE The Man Behind 'Femtochemistry' Ahmed Zewail Wins the 1999 Nobel Prize in Chemistry Puspendu Kumar Das He hails from the land of the Pharaohs; he came to the United States, like many, to seek higher education and today he is at the top of his achievements. He is Ahmed H Zewail, Linus Pauling Chair of Chemical Physics at the California Institute of Tech nology. He has just been honoured with the Nobel Prize in Chemistry by the Royal Swedish Academy of Sciences on Octo ber 12, 1999, 'for his studies of the transition state of chemical Puspendu Kumar Das reactions using femtosecond spectroscopy'. Bond formation and studies and teaches bond breaking are the two most fundamental concepts that physical chemistry at the department of inorganic make chemistry. Zewail studied these two processes using ultra and physical chemistry short techniques on the time scale on which they actually occur. in the Indian Institute of Science, Bangalore. His The Man current research interests include gas Born February 26, 1946 in Egypt, Ahmed H Zewail (Figure 1) phase chemical kinetics, received his BS and MS degrees from Alexandria University second order nonlinear optics, and lipid-protein which was, perhaps, once the greatest place for learning on interaction. earth. He then moved to the University of Pennsylvania, Phila delphia in USA and obtained a PhD degree in 1974 working with R M Hochstrasser. Following his postdoctoral work with Charles Harris at the University of California at Berkeley, he joined the chemistry department of Caltech in 1976. Figure 1. A stamp size He was tenured in two years and became a full portrait (truly) of professor in 1982. -

Femtochemistry and Femtobiology
The desire to explore our surrounding world has always been one of the strongest charac- teristics of human nature. Columbus and the great explorers travelled outwards and discovered new continents in the 16th century and Bohr, Einstein, Rutherford, Curie and many others travelled inward to explore the secrets of the atomic world in the 20th century. Explorers use ships, planes or spacecraft to travel outward and they use increasingly Femtochemistry and Femtobiology (ULTRA) sophisticated scientific tools to travel inward An ESF scientific programme into the world of atoms and molecules. Here at the beginning of the 3rd millennium we have microscopes allowing us to see single atoms and telescopes allowing us to see the edges of the universe. At the end of the 20th century a fascinating new tool, the femtosecond laser, was developed. The femtosecond laser provides us with ULTRA short light pulses, allowing the motion of atoms and molecules to be captured as if they were filmed. To have, not only the structure of atoms and molecules, but also their motion, is of unique importance in our quest to understand and control chemical and biological processes. The ULTRA Programme, sponsored by the European Science Foundation, is a collabo- rative effort among the leading European laboratories engaged in using femtosecond laser pulses in chemistry and biology, a field of research with its own name: Femtochemistry and Femtobiology. In this brochure we give a brief introduction to the The European Science Foundation acts field and illustrate the activities within the as a catalyst Programme with a few illustrative case for the development stories. -

Lasers in Chemistry (CHE00036M) 2020-21 - Module Catalogue, Student Home, University of York Accessibility Statement
10/9/2020 Lasers in Chemistry (CHE00036M) 2020-21 - Module catalogue, Student home, University of York Accessibility statement Lasers in Chemistry - CHE00036M « Back to module search Department: Chemistry Module co-ordinator: Prof. Neil Hunt Credit value: 10 credits Credit level: M Academic year of delivery: 2020-21 See module specification for other years: 2019-20 Module will run Occurrence Teaching cycle A Spring Term 2020-21 to Summer Term 2020-21 Module aims Lasers have become powerful tools in modern chemistry, where they find applications in fields as diverse as astrochemistry, atmospheric remote sensing and analytical research, while the arrival of ultrafast (femtosecond) pulsed lasers has enabled the observation of molecular reactions in real time. The course offers three perspectives on the applications of lasers in chemistry: The first presents an overview of modern lasers and their operation and will discuss how lasers have advanced our understanding in areas ranging from the chemistry of hostile environments to the biomedical arena. The second presents the field of modern laser spectroscopy as applied to the gas phase, focusing on optical spectroscopies of molecular clusters and the use of lasers to measure the fundamental intermolecular interactions that underpin chemistry and biology. The third introduces the field of femtochemistry, discussing how ultrafast lasers can be used to follow chemical processes in the gas and condensed phases in real time. This will include an introduction to advanced spectroscopic methods such as multidimensional spectroscopy and time-resolved electron diffraction. Module learning outcomes Students will gain an understanding of different laser types and understand how lasers are used to both generate and detect key intermediates that control chemistry in combustion processes, in the Earth's atmosphere, and beyond. -

Introduction to Femtochemistry: Excited-State Proton Transfer From
Introduction to Femtochemistry: Excited-State Proton Transfer from Pyranine to Water Studied by Femtosecond Transient Absorption Pascale Changenet, Thomas Gustavsson, Isabelle Lampre To cite this version: Pascale Changenet, Thomas Gustavsson, Isabelle Lampre. Introduction to Femtochemistry: Excited- State Proton Transfer from Pyranine to Water Studied by Femtosecond Transient Absorption. Journal of Chemical Education, American Chemical Society, Division of Chemical Education, 2020, 97 (12), pp.4482-4489. 10.1021/acs.jchemed.0c01056. hal-02991490 HAL Id: hal-02991490 https://hal.archives-ouvertes.fr/hal-02991490 Submitted on 6 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Introduction to Femtochemistry: Excited State Proton Transfer from Pyranine to Water Studied by Femtosecond Transient Absorption Pascale Changenet§*, Thomas Gustavsson†, Isabelle Lampre‡ 5 §Laboratoire d’Optique et Biosciences, CNRS-INSERM-Ecole Polytechnique, Institut Polytechnique de Paris, 91128 Palaiseau, cedex, France †Université Paris-Saclay, CEA-CNRS, Laboratoire Interactions, Dynamiques et Lasers, ERL 9000, 91191 Gif sur Yvette, cedex, France ‡Université Paris-Saclay, CNRS, Institut de Chimie Physique, UMR 8000, 91400 Orsay, 10 France ABSTRACT In order to introduce students to the fascinating field of femtochemistry, we propose here a practical laboratory training course conceived for second-year master students in chemistry.