2. Allotropy in Carbon the Property Due to Which an Element Exists In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Comparison of Sulfur to Oxygen*
OpenStax-CNX module: m34977 1 Comparison of Sulfur to Oxygen* Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Size Table 1 summarizes the comparative sizes of oxygen and sulfur. Element Atomic radius Covalent radius Ionic radius (Å) van der Waal ra- (Å) (Å) dius (Å) Oxygen 0.48 0.66 1.40 1.52 Sulfur 0.88 1.05 1.84 1.80 Table 1: Comparison of physical characteristics for oxygen and sulfur. 2 Electronegativity Sulfur is less electronegative than oxygen (2.4 and 3.5, respectively) and as a consequence bonds to sulfur are less polar than the corresponding bonds to oxygen. One signicant result in that with a less polar S-H bond the subsequent hydrogen bonding is weaker than observed with O-H analogs. A further consequence of the lower electronegativity is that the S-O bond is polar. 3 Bonds formed Sulfur forms a range of bonding types. As with oxygen the -2 oxidation state prevalent. For example, sulfur forms analogs of ethers, i.e., thioethers R-S-R. However, unlike oxygen, sulfur can form more than two covalent (non-dative) bonds, i.e., in compounds such as SF4 and SF6. Such hypervalent compounds were originally thought be due to the inclusion of low energy d orbitals 3 2 in hybrids (e.g., sp d for SF6); however, a better picture involves a combination of s and p ortbitals in bonding (Figure 1). Any involvement of the d orbitals is limited to the polarization of the p orbitals rather than direct hydridization. -
Group Vi Elements (The Chalcogens)
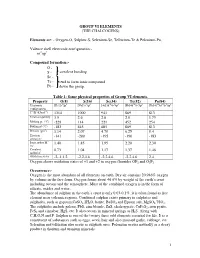
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

The Era of Carbon Allotropes Andreas Hirsch
commentary The era of carbon allotropes Andreas Hirsch Twenty-five years on from the discovery of 60C , the outstanding properties and potential applications of the synthetic carbon allotropes — fullerenes, nanotubes and graphene — overwhelmingly illustrate their unique scientific and technological importance. arbon is the element in the periodic consist of extended networks of sp3- and 1985, with the advent of fullerenes (Fig. 1), table that provides the basis for life sp2 -hybridized carbon atoms, respectively. which were observed for the first time by Con Earth. It is also important for Both forms show unique physical properties Kroto et al.3. This serendipitous discovery many technological applications, ranging such as hardness, thermal conductivity, marked the beginning of an era of synthetic from drugs to synthetic materials. This lubrication behaviour or electrical carbon allotropes. Now, as we celebrate role is a consequence of carbon’s ability conductivity. Conceptually, many other buckminsterfullerene’s 25th birthday, it is to bind to itself and to nearly all elements ways to construct carbon allotropes are also the time to reflect on a growing family in almost limitless variety. The resulting possible by altering the periodic binding of synthetic carbon allotropes, which structural diversity of organic compounds motif in networks consisting of sp3-, sp2- includes the synthesis of carbon nanotubes and molecules is accompanied by a broad and sp-hybridized carbon atoms1,2. As a in 19914 and the rediscovery of graphene range of -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -
Effect of Catenation and Basicity of Pillared Ligand on the Water Stability of Mofs
Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 SUPPORTING INFORMATION (SI) Effect of Catenation and basicity of Pillared Ligand on the Water Stability of MOFs Himanshu Jasuja, and Krista S. Walton* School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Drive NW, Atlanta, Georgia 30332, USA * [email protected] EXPERIMENTAL SECTION 1. Characterization 1.1 PXRD (Powder X-Ray diffraction) patterns 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S1: Comparison of PXRD pattern for as-synthesized Zn-BDC-DABCO or DMOF and its theoretical pattern from single crystal data Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S2: Comparison of PXRD pattern for as-synthesized Zn-BDC-BPY or MOF-508a and its theoretical pattern from single crystal data 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S3: Comparison of PXRD pattern for as-synthesized Zn-TMBDC-DABCO or DMOF-TM and its theoretical pattern from single crystal data Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S4: Comparison of PXRD pattern for as-synthesized Zn-TMBDC-BPY or MOF- 508-TM and its theoretical pattern from single crystal data 6400 3600 Intensity (counts) Intensity 1600 400 0 10 15 20 25 30 35 40 45 2Theta (°) Figure S5: PXRD patterns for as-synthesized Zn-BDC-BPY or MOF-508a and activated Zn-BDC-BPY or MOF-508b displaying shifting of peaks towards right on activation which was also observed by Chen et. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Chemistry Study Materials for Class 10 (NCERT Based: Questions with Answers) Ganesh Kumar Date:- 28/07/2020
Chemistry Study Materials for Class 10 (NCERT Based: Questions with Answers) Ganesh Kumar Date:- 28/07/2020 74. How much carbon is present on earth and CO2 in atmosphere? Answer: 0.02%, 0.03% 75. What is valency of carbon? Answer: 4 76. Why does carbon form strong bonds? Answer: It is due to small size. 77. What will be the product formed when carbon is burnt in presence of air? Answer: Carbon dioxide 78. What is Allotropy? Answer: It is a property due to which an element can exist in more than one form which differ in physical properties but have similar chemical properties, e.g., carbon, sulphur, phosphorus, oxygen show allotropy. 79. Name three allotropes of carbon. Answer: Diamond, graphite and Buckminster fullerenes 80. Which is the purest allotrope of carbon? Answer: Buckminster fullerenes. 81. Why is graphite soft and slippery? Answer: Due to weak vander Waals’ forces of attraction between hexagonal layers. 82. Why is diamond hard? Answer: It is due to strong covalent bonds. 83. Why is diamond lustrous? Answer: It is due to high refractive index. 84. Carbon has four electrons in its valence shell. How does carbon attain stable electronic configuration. Answer: By sharing four electrons with other atoms. 85. Why is carbon tetravalent? Answer: It is because carbon can share form electrons to complete its octet. 86. Which gas is present in LPG? Answer: Butane and Isobutane 87. Which element exhibits the property of catenation to maximum extent and why? Answer: Carbon shows catenation to maximum extent because it forms strong covalent bonds. -

Carbon Nanostructures – from Molecules to Functionalised Materials
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1537 Carbon Nanostructures – from Molecules to Functionalised Materials Fullerene-Ferrocene Oligomers, Graphene Modification and Deposition MICHAEL NORDLUND ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-0019-1 UPPSALA urn:nbn:se:uu:diva-327189 2017 Dissertation presented at Uppsala University to be publicly examined in A1:107a, BMC, Husargatan 3, Uppsala, Friday, 22 September 2017 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Mogens Brøndsted Nielsen (Copenhagen University, Department of chemistry). Abstract Nordlund, M. 2017. Carbon Nanostructures – from Molecules to Functionalised Materials. Fullerene-Ferrocene Oligomers, Graphene Modification and Deposition. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1537. 64 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0019-1. The work described in this thesis concerns development, synthesis and characterisation of new molecular compounds and materials based on the carbon allotropes fullerene (C60) and graphene. A stepwise strategy to a symmetric ferrocene-linked dumbbell of fulleropyrrolidines was developed. The versatility of this approach was demonstrated in the synthesis of a non- symmetric fulleropyrrolidine-ferrocene-tryptophan triad. A new tethered bis-aldehyde, capable of regiospecific bis-pyrrolidination of a C60-fullerene in predominantly trans fashion, was designed, synthesised and reacted with glycine and C60 to yield the desired N-unfunctionalised bis(pyrrolidine)fullerene. A catenane dimer composed of two bis(pyrrolidine)fullerenes was obtained as a minor co-product. From the synthesis of the N-methyl analogue, the catenane dimer could be separated from the monomeric main product and fully characterised by NMR spectroscopy. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

Graphene: a Peculiar Allotrope of Carbon
Graphene: A Peculiar Allotrope Of Carbon Laxmi Nath Bhattarai Department of Physics, Butwal Multiple Campus Correspondence: [email protected] Abstract Graphene is a two dimensional one atom thick allotrope of Carbon. Electrons in grapheme behave as massless relativistic particles. It is a 2 dimensional nanomaterial with many peculiar properties. In grapheme both integral and fractional quantum Hall effects are observed. Many practical application are seen from use of Graphene material. Keywords: Graphene, allotrope, Quantum Hall effect, nanomaterial. Introduction Graphene is a two dimensional allotropic form of Honey comb structure of Graphene carbon. This was discovered in 2004. Diamond and Graphene is considered as the mother of all graphitic Graphite are three dimensional allotropes of carbon materials because it is the building block of carbon which are known from ancient time. One dimensional materials of all other dimensions (Srinivasan, 2007). carbon nanotubes were discovered in 1991 and Griphite is obtained by the stacking of Graphene zero dimensional Fullerenes were discovered in layers, Diamond can be obtained from Graphene under 1985. Graphene was experimentally extracted from extreme pressure and temperatures. One-dimensional 3-dimensional Graphite by Physisists Andre Geim and carbon nanotubes can be obtained by rolling and Konstantin Novoselov of Manchester University UK Zero–dimensional Fullerenes is obtained by wrapping in 2004. For their remarkable work they were awarded Graphene layers. the Nobel Prize of Physics for the year 2010. Properties Structure The young material Graphene is found to have Graphene is a mono layer of Carbon atoms packed following unique properties: into a honeycomb crystal structure. Graphene sheets 2 are one atom thick 2-dimensional layers of sp – bonded a) It is the thinnest material in the universe and the carbon. -

Carbon-Based Nanomaterials/Allotropes: a Glimpse of Their Synthesis, Properties and Some Applications
materials Review Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications Salisu Nasir 1,2,* ID , Mohd Zobir Hussein 1,* ID , Zulkarnain Zainal 3 and Nor Azah Yusof 3 1 Materials Synthesis and Characterization Laboratory (MSCL), Institute of Advanced Technology (ITMA), Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia 2 Department of Chemistry, Faculty of Science, Federal University Dutse, 7156 Dutse, Jigawa State, Nigeria 3 Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; [email protected] (Z.Z.); [email protected] (N.A.Y.) * Correspondence: [email protected] (S.N.); [email protected] (M.Z.H.); Tel.: +60-1-2343-3858 (M.Z.H.) Received: 19 November 2017; Accepted: 3 January 2018; Published: 13 February 2018 Abstract: Carbon in its single entity and various forms has been used in technology and human life for many centuries. Since prehistoric times, carbon-based materials such as graphite, charcoal and carbon black have been used as writing and drawing materials. In the past two and a half decades or so, conjugated carbon nanomaterials, especially carbon nanotubes, fullerenes, activated carbon and graphite have been used as energy materials due to their exclusive properties. Due to their outstanding chemical, mechanical, electrical and thermal properties, carbon nanostructures have recently found application in many diverse areas; including drug delivery, electronics, composite materials, sensors, field emission devices, energy storage and conversion, etc. Following the global energy outlook, it is forecasted that the world energy demand will double by 2050. This calls for a new and efficient means to double the energy supply in order to meet the challenges that forge ahead. -

Group 13 Elements
GROUP I5 ELEMENTS Q. 1. Give chemical reaction in support of the statement that all the bonds in PCI5 molecule are not equivalent. Ans. Due to greater bond pair-bond pair repulsions, the two axial P — Cl bonds are less stable than the three equatorial P — Cl bonds. It is because of this reason that when PC15 is heated, the less stable axial bonds are Broken to form Cl2. Δ PCl5 -------------- PCl3 + Cl2. Q. 2. The tendency for catenation decreases down the group 14. Or Why carbon shows catenation but silicon does not Ans. As we move down the group 14, the atomic size increases and hence the strength of M—M bond decreases steadily. Consequently the tendency for catenation decreases down the group. Q. 3. PCI5 is known but NC15 is not known. Explain. 2 2 6 2 1 1 1 Ans. Electronic configuration of P is 1s 2s 2p 3s 3px 3py 3pz . Thus phosphorus has empty 3d orbitals in which the elecron of the 3s-orbital can be excited but nitrogen has no d-orbitals as its electronic configuration is 2 2 1 1 1 1s 2s 2px 2py 2pz . Q. 4. Why molecular nitrogen is not reactive ? Ans. The bond dissociation energy for the triple bond in N = N is very high because of small size of N-atoms and hence small internuclear distance. Q.5. H3PO3 is diprotic (or)dibasic. Why? Ans. Since it has only two ionisable H – atoms which are present as OH groups. Q. 6. Nitrogen exists as diatomic molecule, N2, whereas phosphorus exists a tetratomic molecule P4.