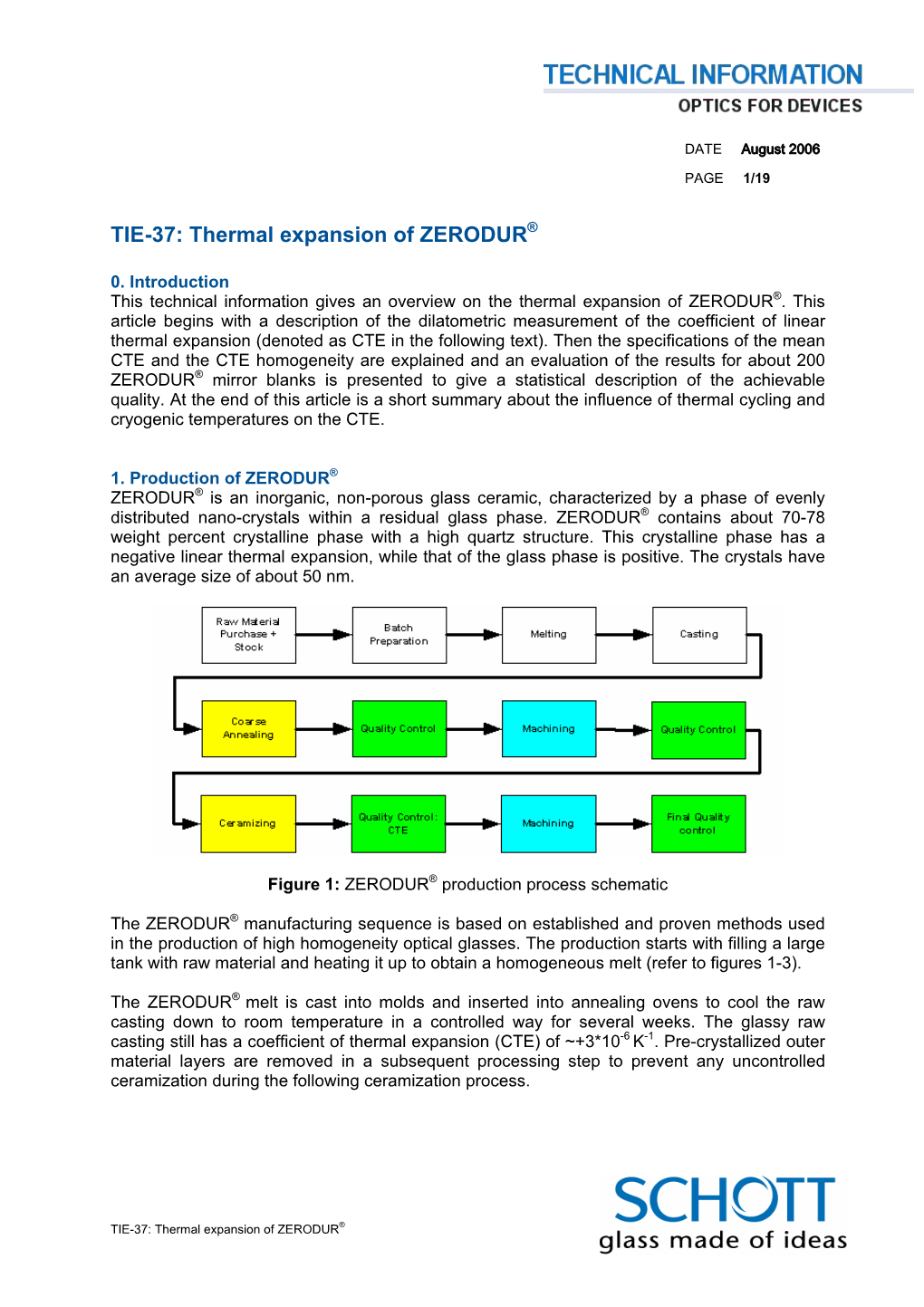
TIE-37: Thermal Expansion of ZERODUR®
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photonic Glass-Ceramics: Consolidated Outcomes and Prospects Brigitte Boulard1, Tran T
Photonic glass-ceramics: consolidated outcomes and prospects Brigitte Boulard1, Tran T. T. Van2, Anna Łukowiak3, Adel Bouajaj4, Rogéria Rocha Gonçalves5, Andrea Chiappini6, Alessandro Chiasera6, Wilfried Blanc7, Alicia Duran8, Sylvia Turrell9, Francesco Prudenzano10, Francesco Scotognella11, Roberta Ramponi11, Marian Marciniak12, Giancarlo C. Righini13,14, Maurizio Ferrari6,13,* 1 Institut des Molécules et Matériaux du Mans, UMR 6283, Equipe Fluorures, Université du Maine, Av. Olivier Messiaen, 72085 Le Mans cedex 09, France. 2 University of Science Ho Chi Minh City, 227 Nguyen Van Cu, Dist.5, HCM Vietnam. 3 Institute of Low Temperature and Structure Research, PAS, ul. Okolna 2, 50-950 Wroclaw, Poland. 4 Laboratory of innovative technologies, LTI, ENSA–Tangier, University Abdelmalek Essaâdi, Tangier, Morocco. 5 Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo - Av. Bandeirantes, 3900, CEP 14040-901, Ribeirão Preto/SP, Brazil 6 CNR-IFN, CSMFO Lab., Via alla Cascata 56/c, Povo, 38123 Trento, Italy. 7 Université Nice Sophia Antipolis, CNRS LPMC, UMR 7336, 06100 Nice, France. 8 Instituto de Ceramica y Vidrio (CSIC), C/Kelsen 5, Campus de Cantoblanco, 28049 Madrid, Spain. 9 LASIR (CNRS, UMR 8516) and CERLA, Université Lille 1, 59650 Villeneuve d’Ascq, France. 10 Politecnico di Bari, DEI, Via E. Orabona 4, Bari, 70125, Italy. 11 IFN-CNR and Department of Physics, Politecnico di Milano, p.zza Leonardo da Vinci 32, 20133 Milano, Italy 12 National Institute of Telecommunications, 1 Szachowa Street, 04 894 Warsaw, Poland. 13 Centro di Studi e Ricerche “Enrico Fermi”, Piazza del Viminale 2, 00184 Roma, Italy. 14 MipLAB. IFAC - CNR, Via Madonna del Piano 10, 50019 Sesto Fiorentino, Italy. -

The American Ceramic Society 25Th International Congress On
The American Ceramic Society 25th International Congress on Glass (ICG 2019) ABSTRACT BOOK June 9–14, 2019 Boston, Massachusetts USA Introduction This volume contains abstracts for over 900 presentations during the 2019 Conference on International Commission on Glass Meeting (ICG 2019) in Boston, Massachusetts. The abstracts are reproduced as submitted by authors, a format that provides for longer, more detailed descriptions of papers. The American Ceramic Society accepts no responsibility for the content or quality of the abstract content. Abstracts are arranged by day, then by symposium and session title. An Author Index appears at the back of this book. The Meeting Guide contains locations of sessions with times, titles and authors of papers, but not presentation abstracts. How to Use the Abstract Book Refer to the Table of Contents to determine page numbers on which specific session abstracts begin. At the beginning of each session are headings that list session title, location and session chair. Starting times for presentations and paper numbers precede each paper title. The Author Index lists each author and the page number on which their abstract can be found. Copyright © 2019 The American Ceramic Society (www.ceramics.org). All rights reserved. MEETING REGULATIONS The American Ceramic Society is a nonprofit scientific organization that facilitates whether in print, electronic or other media, including The American Ceramic Society’s the exchange of knowledge meetings and publication of papers for future reference. website. By participating in the conference, you grant The American Ceramic Society The Society owns and retains full right to control its publications and its meetings. -

Glossary of Terms Abrisa Technologies Your Single Source Optics Partner!
Glossary of Terms Abrisa Technologies Your Single Source Optics Partner! December 2015 200 South Hallock Drive, Santa Paula, CA 93060 • (877) 622-7472 • FAX (805) 525-8604 • www.abrisatechnologies.com Glossary of Terms - 12/15 2 of 13 Acid Etching This process for the decoration of glass involves the application of hydrofluoric acid to the glass surface. Hydrofluoric acid vapors or baths of hydrofluoric acid salts may be used to give glass a matte, frosted appearance (similar to that obtained by surface sandblasting), as found in lighting glass. Glass designs can be produced by coating the glass with wax and then inscribing the desired pattern through the wax layer. When applied, the acid will corrode the glass but not attack the wax-covered areas. Alumina-silicate Glass Alumina (aluminum oxide Al2O3) is added to the glass batch in the form of commonly found feldspars containing alkalis in order to help improve chemical resistance and mechanical strength, and to increase viscosity at lower temperatures. Angle of Incidence The angle formed between a ray of light striking a surface and the normal line (the line perpendicular to the surface at that point). Annealing Under natural conditions, the surface of molten glass will cool more rapidly than the center. This results in internal stress- es which may cause the glass sheet or object to crack, shatter or even explode some time later. The annealing process is designed to eliminate or limit such stresses by submitting the glass to strictly controlled cooling in a special oven known as a “lehr”. Inside the lehr, the glass is allowed to cool to a temperature known as the “annealing point”. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School ADVENTURES IN HIGH DIMENSIONS: UNDERSTANDING GLASS FOR THE 21ST CENTURY A Dissertation in Material Science and Engineering by Collin James Wilkinson © 2021 Collin James Wilkinson Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2021 ii The dissertation of Collin Wilkinson was reviewed and approved by the following: John Mauro Professor of Materials Science and Engineering Chair, Intercollege Graduate Degree Program Associate Head for Graduate Education, Materials Science and Engineering Dissertation Advisor Chair of Committee Seong Kim Professor of Chemical Engineering Professor of Materials Science and Engineering Ismaila Dabo Associate Professor of Materials Science and Engineering Susan Sinnott Professor of Materials Science and Engineering Professor of Chemistry Head of the Department of Materials Science and Engineering iii Abstract Glass is infinitely variable. This complexity stands as a promising technology for the 21st century since the need for environmentally friendly materials has reached a critical point due to climate change. However, such a wide range of variability makes new glass compositions difficult to design. The difficulty is only exaggerated when considering that not only is there an infinite variability in the compositional space, but also an infinite variability thermal history of a glass and in the crystallinity of glass-cearmics. This means that even for a simple binary glass there are at least 3 dimensions that have to be optimized. To resolve this difficulty, it is shown that energy landscapes can capture all three sets of complexity (composition, thermal history, and crystallinity). The explicit energy landscape optimization, however, has a large computational cost. -

Fabrication of Porous Bioactive Glass-Ceramics Via Decomposition of Natural Fibres
Journal of Metals, Materials and Minerals. Vol.18 No.2 pp.85-91, 2008 Fabrication of Porous Bioactive Glass-Ceramics via Decomposition of Natural Fibres Pat SOOKSAEN1*, Supakij SUTTIRUENGWONG1,2, Kunwadee ONIEM1, Khanamporn NGAMLAMIAD1 and Jitlada ATIREKLAPWARODOM1 1Department of Materials Science and Engineering, Faculty of Engineering and Industrial Technology, Silpakorn University, Nakhon Pathom 73000, Thailand 2Center of Excellence for Petroleum, Petrochemicals and Advanced Materials, Silpakorn University, Nakhon Pathom 73000, Thailand Received Sept. 22, 2008 Abstract Accepted Oct. 27, 2008 Porous bio glass-ceramics were prepared via natural fibres burning-out process. Glass-ceramics were fabricated by controlled crystallization of suitable glass compositions to give required crystalline phase/s. Porous structure was formed alongside during burning-out of natural fibres. Three glass batches were prepared in the SiO2-CaO-P2O5-K2O-Na2O-CaF2 glass system, then melted, quenched and milled to give fine glass powders. DTA thermograms for the three glass compositions showed crystallization temperatures between 720 and 760°C and Tg ranging between 508 and 645°C. Porous glass-ceramics were characterized for bulk density, phase evolution and microstructures. Different compositions resulted in different bulk densities and phases that crystallized out. Fluorapatite was found in all glass-ceramic samples, which indicated the biocompatibility and bioactivity and the potential future applications. Microstructures were also different from different -

Lecture #16 Glass-Ceramics: Nature, Properties and Processing Edgar Dutra Zanotto Federal University of São Carlos, Brazil [email protected] Spring 2015
Glass Processing Lecture #16 Glass-ceramics: Nature, properties and processing Edgar Dutra Zanotto Federal University of São Carlos, Brazil [email protected] Spring 2015 Lectures available at: www.lehigh.edu/imi Sponsored by US National Science Foundation (DMR-0844014) 1 Glass-ceramics: nature, applications and processing (2.5 h) 1- High temperature reactions, melting, homogeneization and fining 2- Glass forming: previous lectures 3- Glass-ceramics: definition & applications (March 19) Today, March 24: 4- Composition and properties - examples 5- Thermal treatments – Sintering (of glass powder compactd) or -Controlled nucleation and growth in the glass bulk 6- Micro and nano structure development April 16 7- Sophisticated processing techniques 8- GC types and applications 9- Concluding remmarks 2 Review of Lecture 15 Glass-ceramics -Definition -History -Nature, main characteristics -Statistics on papers / patents - Properties, thermal treatments micro/ nanostructure design 3 Reading assignments E. D. Zanotto – Am. Ceram. Soc. Bull., October 2010 Zanotto 4 The discovery of GC Natural glass-ceramics, such as some types of obsidian “always” existed. René F. Réaumur – 1739 “porcelain” experiments… In 1953, Stanley D. Stookey, then a young researcher at Corning Glass Works, USA, made a serendipitous discovery ...… 5 <rms> 1nm Zanotto 6 Transparent GC for domestic uses Zanotto 7 Company Products Crystal type Applications Photosensitive and etched patterned Foturan® Lithium-silicate materials SCHOTT, Zerodur® β-quartz ss Telescope mirrors Germany -

New Glass Review 10.Pdf
'New Glass Review 10J iGl eview 10 . The Corning Museum of Glass NewG lass Review 10 The Corning Museum of Glass Corning, New York 1989 Objects reproduced in this annual review Objekte, die in dieser jahrlich erscheinenden were chosen with the understanding Zeitschrift veroffentlicht werden, wurden unter that they were designed and made within der Voraussetzung ausgewahlt, dal3 sie the 1988 calendar year. innerhalb des Kalenderjahres 1988 entworfen und gefertigt wurden. For additional copies of New Glass Review, Zusatzliche Exemplare des New Glass Review please contact: konnen angefordert werden bei: The Corning Museum of Glass Sales Department One Museum Way Corning, New York 14830-2253 (607) 937-5371 All rights reserved, 1989 Alle Rechtevorbehalten, 1989 The Corning Museum of Glass The Corning Museum of Glass Corning, New York 14830-2253 Corning, New York 14830-2253 Printed in Dusseldorf FRG Gedruckt in Dusseldorf, Bundesrepublik Deutschland Standard Book Number 0-87290-119-X ISSN: 0275-469X Library of Congress Catalog Card Number Aufgefuhrt im Katalog der KongreB-Bucherei 81-641214 unter der Nummer 81-641214 Table of Contents/lnhalt Page/Seite Jury Statements/Statements der Jury 4 Artists and Objects/Kunstler und Objekte 10 Bibliography/Bibliographie 30 A Selective Index of Proper Names and Places/ Verzeichnis der Eigennamen und Orte 53 er Wunsch zu verallgemeinern scheint fast ebenso stark ausgepragt Jury Statements Dzu sein wie der Wunsch sich fortzupflanzen. Jeder mochte wissen, welchen Weg zeitgenossisches Glas geht, wie es in der Kunstwelt bewer- tet wird und welche Stile, Techniken und Lander maBgeblich oder im Ruckgang begriffen sind. Jedesmal, wenn ich mich hinsetze und einen Jurybericht fur New Glass Review schreibe (dies ist mein 13.), winden he desire to generalize must be almost as strong as the desire to und krummen sich meine Gedanken, um aus den tausend und mehr Dias, Tprocreate. -

Vitrification of Historic and Future High Level Nuclear Wastes Within Alkali Borosilicate Glasses
Vitrification of historic and future high level nuclear wastes within alkali borosilicate glasses Andrew James Connelly M.Eng. A Thesis submitted to the Department of Engineering Materials at the University of Sheffield in partial fulfilment of the requirement for the Degree of Doctor of Philosophy. February 2008 The University Of Sheffield. Abstract The disposal of highly radioactive and toxic wastes generated by the nuclear industry is one of the biggest challenges facing the world today. Currently, in the UK there is a large legacy waste holding which has been accumulating since nuclear energy was first harnessed during World War 2. Processing of this waste with a view to final disposal is a complex and difficult task. This work investigates one aspect of that process, namely turning this waste into glass (or vitrification). This work uses multiple techniques including x-ray absorption spectroscopy, magic angle spinning nuclear magnetic resonance and molecular dynamic simulations, to investigate the structural role of Zr02 and U 03 within the alkali borosilicate glass used in the UK for waste immobilisation. The effect of these additions on the bulk glass structure and selected glass properties are also explored. In waste glasses Zr occurs as a 6 co-ordinated Zr ion surrounded by Si, B, Na and Li. The effect of Zr02 additions on the bulk glass structure and properties is highly complex. The addition of Zr02 appears to be characterised by a non-linearity in the trends of certain physical and structural parameters. At low levels of Zr02 the level of leaching from the glasses and the co ordination of B increase. -

Technical Glasses
Technical Glasses Physical and Technical Properties 2 SCHOTT is an international technology group with 130 years of ex perience in the areas of specialty glasses and materials and advanced technologies. With our highquality products and intelligent solutions, we contribute to our customers’ success and make SCHOTT part of everyone’s life. For 130 years, SCHOTT has been shaping the future of glass technol ogy. The Otto Schott Research Center in Mainz is one of the world’s leading glass research institutions. With our development center in Duryea, Pennsylvania (USA), and technical support centers in Asia, North America and Europe, we are present in close proximity to our customers around the globe. 3 Foreword Apart from its application in optics, glass as a technical ma SCHOTT Technical Glasses offers pertinent information in terial has exerted a formative influence on the development concise form. It contains general information for the deter of important technological fields such as chemistry, pharma mination and evaluation of important glass properties and ceutics, automotive, optics, optoelectronics and information also informs about specific chemical and physical character technology. Traditional areas of technical application for istics and possible applications of the commercial technical glass, such as laboratory apparatuses, flat panel displays and glasses produced by SCHOTT. With this brochure, we hope light sources with their various requirements on chemical to assist scientists, engineers, and designers in making the physical properties, have led to the development of a great appropriate choice and make optimum use of SCHOTT variety of special glass types. Through new fields of appli products. cation, particularly in optoelectronics, this variety of glass types and their modes of application have been continually Users should keep in mind that the curves or sets of curves enhanced, and new forming processes have been devel shown in the diagrams are not based on precision measure oped. -

V 08916 S.Pdf
Annotation The objective of this thesis is to describe in details the process of bonding of ZERODUR mirrors on CNC holder, and the influence of the heating and cooling of the wax on the final shape of mirror. For bonding of the ZERODUR glass to the aluminum holder we have used red wax, and we have monitor the influence of this material to the final shape of our optic by measuring the surface shape before and after bonding by using the LuphoScan. Furthermore the consecutive process of bonding numerically is verified by using MSC Marc& MSC Mentat 2015. This work shows that for manufacturing of ultra-precise optics bonding of the ZERODUR glass to the ZERODUR disk gives better results, compared to aluminum disk. Keywords: visco- elastic, precision machining, grinding, polishing, heat transfer, experiments, FEM 6 Table of Contents Annotation........................................................................................................................................6 Table of Contents .............................................................................................................................7 List of Tables ...................................................................................................................................9 List of Figures ................................................................................................................................10 List of Symbols ..............................................................................................................................12 -

Properties and Applications of Porous Glasses from Foamed Glasses and Gel-Derived Glasses to Allophanes
MATERIA CONDENSADA REVISTA MEXICANA DE FISICA´ S 52 (3) 190–194 MAYO 2006 Properties and applications of porous glasses from foamed glasses and gel-derived glasses to allophanes T. Woigniera;b;¤, Adil Hafidi Alaouib, Juan Primeraa;c, and Jean Phalippoua aLaboratoire des collo¨ıdes, verres et nanocomposite du CNRS, Montpellier, France bIRD -Pole de Recherche Agronomique de la Martinique, Le Lamentin, France cDepartamento de fisica, FEC, LUZ. Maracaibo, Venezuela Recibido el 24 de noviembre de 2003; aceptado el 12 de octubre de 2004 Porous glasses can be synthesised by different methods: in the conventional ways such as foaming (foamed glasses) or phase separation and leaching process leading to the Vycor glass, and also by the sol-gel method (sintered xerogel and aerogel), or by geological alteration process of volcanic materials (allophanes). In this paper we will discuss different features of these porous materials related to their properties, and their possible applications such as biomaterials (foamed glasses), host matrices for nuclear wastes (Vycor and sintered gels), precursors for special glasses (xerogels and aerogels), and mitigation of the greenhouse gases by carbon sequestration (allophanes). Keywords: Porous glasses, sol gel process, allophanes. Los vidrios porosos pueden sintetizarse por diferentes v´ıas: por las tradicionales, tales como el espumado (vidrios espumosos) o la separacion´ de fase seguida de lixiviacion,´ (con lo que se produce el vidrio Vycor), y tambien´ mediante otras de desarrollo mas´ reciente como el metodo´ sol-gel (geles) o de procesos de alteracion´ geologicas´ de materiales volcanicos´ (alofanos). En este art´ıculo discutimos diferentes caracter´ısticas de estos materiales porosos y la relacion´ de estas´ con sus propiedades y las posibles aplicaciones como biomateriales (vidrios espumosos), matrices de almacenamiento para desechos nucleares (Vycor y geles), precursores de vidrios especiales (xerogeles y aerogeles), y en la disminucion´ del efecto invernadero por secuestracion´ de carbon´ (alofanos). -

Borosilicate Glass Wafers Are an Inexpensive Alternative
Basics of Microstructuring 01 Chapter MicroChemicals® – Fundamentals of Microstructuring www.microchemicals.com/downloads/application_notes.html PRODUCTION AND SPECIFICATIONS OF GLASS WAFERS For applications where neither the high dielectric strength of quartz nor the high transparency is in the ultravi- olet, visible or infrared spectral range or the thermal stability of quartz or quartz glass is required, borosilicate glass wafers are an inexpensive alternative. Borosilicate Glass and Ordinary Glass in Comparison Composition Borosilicate glass consists of approximately 80% of silicon dioxide (SiO2) and approximately 5-15% boron trioxide (B2O3). Other additives are alkaline oxide (Na2O, K2O), aluminium oxide (Al2O3) and alkaline po- tassium oxide (CaO, MgO). Borosilicate glasses typically have a very low iron content, which causes the typical green colour of window glass in order to increase the transparency. Properties Due to its boron content, borosilicate glass exhibit a higher chemical stability against water, many chemi- cals and pharmaceutical products compared to window glass. In addition, borosilicate glass exhibits signifi cantly higher thermal stability against temperature fl uctua- tions due to its thermal expansion coeffi cients, which are less than half as large as compared to window glass. Production of Glass Wafers For the further processing into glass wafers, specifi c borosilicate glass is produced using the “fl oat pro- cess” (Fig. 37). Hereby the molten glass on melted tin forms a fl oating both-side smooth ribbon with a homogeneous thickness. On its way on the tin bath, the temperature is gradually reduced from 1100 down to 600°C until the sheet can be lifted onto rollers. Glass pane Glass melt Tin melt Fig.