Quarantine Evaluation of Eucryptorrhynchus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Illinois Native Plant Society 2019 Plant List Herbaceous Plants
Illinois Native Plant Society 2019 Plant List Plant Sale: Saturday, May 11, 9:00am – 1:00pm Illinois State Fairgrounds Commodity Pavilion (Across from Grandstand) Herbaceous Plants Scientific Name Common Name Description Growing Conditions Comment Dry to moist, Sun to part Blooms mid-late summer Butterfly, bee. Agastache foeniculum Anise Hyssop 2-4', Lavender to purple flowers shade AKA Blue Giant Hyssop Allium cernuum var. Moist to dry, Sun to part Nodding Onion 12-18", Showy white flowers Blooms mid summer Bee. Mammals avoid cernuum shade 18", White flowers after leaves die Allium tricoccum Ramp (Wild Leek) Moist, Shade Blooms summer Bee. Edible back Moist to dry, Part shade to Blooms late spring-early summer Aquilegia canadensis Red Columbine 30", Scarlet and yellow flowers shade Hummingbird, bee Moist to wet, Part to full Blooms late spring-early summer Showy red Arisaema dracontium Green Dragon 1-3', Narrow greenish spadix shade fruits. Mammals avoid 1-2', Green-purple spadix, striped Moist to wet, Part to full Blooms mid-late spring Showy red fruits. Arisaema triphyllum Jack-in-the-Pulpit inside shade Mammals avoid Wet to moist, Part shade to Blooms late spring-early summer Bee. AKA Aruncus dioicus Goatsbeard 2-4', White fluffy panicles in spring shade Brides Feathers Wet to moist, Light to full Blooms mid-late spring Mammals avoid. Asarum canadense Canadian Wildginger 6-12", Purplish brown flowers shade Attractive groundcover 3-5', White flowers with Moist, Dappled sun to part Blooms summer Monarch larval food. Bee, Asclepias exaltata Poke Milkweed purple/green tint shade butterfly. Uncommon Blooms mid-late summer Monarch larval Asclepias hirtella (Tall) Green Milkweed To 3', Showy white flowers Dry to moist, Sun food. -

Chicago Joins New York in Battle with the Asian Longhorned Beetle Therese M
Chicago Joins New York in Battle with the Asian Longhorned Beetle Therese M. Poland, Robert A. Haack, Toby R. Petrice USDA Forest Service, North Central Research Station, 1407 S. Harrison Rd., Rm. 220, E. Lansing, MI 48823 The Asian longhorned beetle, Anoplophora glabripennis (Motschulsky), was positively iden- would follow New York’s lead tified on 13 July 1998 attacking trees in an area of and that infested trees would northern Chicago known as Ravenswood. Previ- be cut, chipped, burned and ously, the only known North American occur- replaced by new trees at the rence of this Asian cerambycid beetle was in the city’s expense. Amityville area and the Brooklyn area of Long The city of Chicago ben- Island, New York, where it was discovered in efited greatly from New August 1996 (Haack et al. 1996, Cavey et al. York’s experience in imple- 1998). In New York, this woodborer has attacked menting its eradication program. With an excellent species of maple (Acer), horsechestnut (Aesculus well as 1 square mile each in Addison and in leadership team and organization, the city of hippocastanum), birch (Betula), poplar (Populus), Summit. Extensive surveys were conducted out Chicago obtained public cooperation and support willow (Salix), and elm (Ulmus) (Haack et al. to 1 ¼ miles past the outer boundary of known for the eradication program from the outset. The 1997). Because of the potential for longterm infested trees at all three locations. Survey crews media provided excellent, factual and accurate ecological and economic damage an aggressive were composed of APHIS inspectors, federal, information through extensive television, newspa- eradication program that involves locating, re- state and city employees as well as APHIS trained per, and radio coverage. -

Oregon Invasive Species Action Plan
Oregon Invasive Species Action Plan June 2005 Martin Nugent, Chair Wildlife Diversity Coordinator Oregon Department of Fish & Wildlife PO Box 59 Portland, OR 97207 (503) 872-5260 x5346 FAX: (503) 872-5269 [email protected] Kev Alexanian Dan Hilburn Sam Chan Bill Reynolds Suzanne Cudd Eric Schwamberger Risa Demasi Mark Systma Chris Guntermann Mandy Tu Randy Henry 7/15/05 Table of Contents Chapter 1........................................................................................................................3 Introduction ..................................................................................................................................... 3 What’s Going On?........................................................................................................................................ 3 Oregon Examples......................................................................................................................................... 5 Goal............................................................................................................................................................... 6 Invasive Species Council................................................................................................................. 6 Statute ........................................................................................................................................................... 6 Functions ..................................................................................................................................................... -
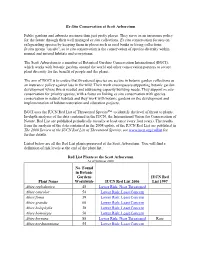
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

First Phylogeny of Bitterbush Family, Picramniaceae (Picramniales)
plants Article First Phylogeny of Bitterbush Family, Picramniaceae (Picramniales) Alexey Shipunov 1,*, Shyla Carr 1, Spencer Furniss 1, Kyle Pay 1 and José Rubens Pirani 2 1 Minot State University, Minot, ND 58707, USA; [email protected] (S.C.); [email protected] (S.F.); [email protected] (K.P.) 2 University of São Paulo, São Paulo 01000-000, Brazil; [email protected] * Correspondence: [email protected] Received: 17 December 2019; Accepted: 19 February 2020; Published: 21 February 2020 Abstract: Picramniaceae is the only member of Picramniales which is sister to the clade (Sapindales (Huerteales (Malvales, Brassicales))) in the rosidsmalvids. Not much is known about most aspects of their ecology, geography, and morphology. The family is restricted to American tropics. Picramniaceae representatives are rich in secondary metabolites; some species are known to be important for pharmaceutical purposes. Traditionally, Picramniaceae was classified as a subfamily of Simaroubaceae, but from 1995 on, it has been segregated containing two genera, Picramnia and Alvaradoa, with the recent addition of a third genus, Nothotalisia, described in 2011. Only a few species of the family have been the subject of DNA-related research, and fewer than half of the species have been included in morphological phylogenetic analyses. It is clear that Picramniaceae remains a largely under-researched plant group. Here we present the first molecular phylogenetic tree of the group, based on both chloroplast and nuclear markers, widely adopted in the plant DNA barcoding. The main findings are: The family and its genera are monophyletic and Picramnia is sister to two other genera; some clades corroborate previous assumptions of relationships made on a morphological or geographical basis, while most parts of the molecular topology suggest high levels of homoplasy in the morphological evolution of Picramnia. -

Plants from the Brazilian Traditional Medicine: Species from the Books Of
Revista Brasileira de Farmacognosia 27 (2017) 388–400 ww w.elsevier.com/locate/bjp Original Article Plants from the Brazilian Traditional Medicine: species from the books of the Polish physician Piotr Czerniewicz (Pedro Luiz Napoleão Chernoviz, 1812–1881) a,c d b,c b,c,∗ Letícia M. Ricardo , Juliana de Paula-Souza , Aretha Andrade , Maria G.L. Brandão a Ministério da Saúde, Departamento de Assistência Farmacêutica e Insumos Estratégicos, Esplanada dos Ministérios, Brasília, DF, Brazil b Centro Especializado em Plantas Aromáticas, Medicinais e Tóxicas, Museu de História Natural e Jardim Botânico, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil c Faculdade de Farmácia, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil d Universidade Federal de São João del Rei, Campus Sete Lagoas, Sete Lagoas, MG, Brazil a b s t r a c t a r t i c l e i n f o Article history: The Brazilian flora is very rich in medicinal plants, and much information about the traditional use of Received 1 October 2016 the Brazilian plants is only available from early literature and we are facing a rapid process of loss of Accepted 10 January 2017 biodiversity. To retrieve data about useful plants registered in the books of the Polish physicist P.L.N. Available online 2 March 2017 Chernoviz, who lived in Brazil for 15 years in the 19th century. The aim is to improve our knowledge about Brazilian plants, and to ensure the benefits of sharing it with potential users. Data about Brazilian Keywords: plants were obtained from six editions of the book Formulary and Medical Guide (Formulário e Guia Historical records Médico), published in 1864, 1874, 1888, 1892, 1897 and 1920. -

Tree Height Growth in a Neotropical Rain Forest
Ecology, 82(5), 2001, pp. 1460±1472 q 2001 by the Ecological Society of America GETTING TO THE CANOPY: TREE HEIGHT GROWTH IN A NEOTROPICAL RAIN FOREST DEBORAH A. CLARK1 AND DAVID B. CLARK1 Department of Biology, University of Missouri±St. Louis, 8001 Natural Bridge Road, St. Louis, Missouri 63121-4499, USA Abstract. There is still limited understanding of the processes underlying forest dy- namics in the world's tropical rain forests, ecosystems of disproportionate importance in terms of global biogeochemistry and biodiversity. Particularly poorly documented are the nature and time scale of upward height growth during regeneration by the tree species in these communities. In this study, we assessed long-term height growth through ontogeny for a diverse group of canopy and emergent tree species in a lowland neotropical rain forest (the La Selva Biological Station, northeastern Costa Rica). Species were evaluated based on annual height measurements of large samples of individuals in all postseedling size classes, over a 16-yr period (.11 000 increments). The study species were seven nonpi- oneers (Minquartia guianensis, Lecythis ampla, Hymenolobium mesoamericanum, Sima- rouba amara, Dipteryx panamensis, Balizia elegans, and Hyeronima alchorneoides) and two pioneers (Cecropia obtusifolia and Cecropia insignis). For each species, inherent height growth capacity was estimated as the mean of the ®ve largest annual height increments (from different individuals) in each juvenile size class (from 50 cm tall to 20 cm in diameter). All species showed marked ontogenetic increases in this measure of height growth potential. At all sizes, there were highly signi®cant differences among species in height growth potential. -

Weevils) of the George Washington Memorial Parkway, Virginia
September 2020 The Maryland Entomologist Volume 7, Number 4 The Maryland Entomologist 7(4):43–62 The Curculionoidea (Weevils) of the George Washington Memorial Parkway, Virginia Brent W. Steury1*, Robert S. Anderson2, and Arthur V. Evans3 1U.S. National Park Service, 700 George Washington Memorial Parkway, Turkey Run Park Headquarters, McLean, Virginia 22101; [email protected] *Corresponding author 2The Beaty Centre for Species Discovery, Research and Collection Division, Canadian Museum of Nature, PO Box 3443, Station D, Ottawa, ON. K1P 6P4, CANADA;[email protected] 3Department of Recent Invertebrates, Virginia Museum of Natural History, 21 Starling Avenue, Martinsville, Virginia 24112; [email protected] ABSTRACT: One-hundred thirty-five taxa (130 identified to species), in at least 97 genera, of weevils (superfamily Curculionoidea) were documented during a 21-year field survey (1998–2018) of the George Washington Memorial Parkway national park site that spans parts of Fairfax and Arlington Counties in Virginia. Twenty-three species documented from the parkway are first records for the state. Of the nine capture methods used during the survey, Malaise traps were the most successful. Periods of adult activity, based on dates of capture, are given for each species. Relative abundance is noted for each species based on the number of captures. Sixteen species adventive to North America are documented from the parkway, including three species documented for the first time in the state. Range extensions are documented for two species. Images of five species new to Virginia are provided. Keywords: beetles, biodiversity, Malaise traps, national parks, new state records, Potomac Gorge. INTRODUCTION This study provides a preliminary list of the weevils of the superfamily Curculionoidea within the George Washington Memorial Parkway (GWMP) national park site in northern Virginia. -

2019 Goose Creek Native Revegetation and Restoration
2019 Goose Creek Native Revegetation and Restoration Written by: Joe Dahlke, Katie Fischer, Michaela Fishback, Marissa Lane-Masse, and Steven Pearlman Riparian Restoration Environmental Leadership Program Conducted by the University of Oregon’s 2019 Environmental Leadership Program Website: https://blogs.uoregon.edu/2019riparianrestoration/ Table of Contents 1.0 Executive Summary……………………………………………………………………….....2 2.0 Introduction: About the Project…….……………………………………………...……….2 2.1 History and Background of Whitewater Ranch…………………………….…………2 2.2 Study Area…………………………………………………………………………….3 3.0 Stewardship Summary……………………………………………………………………....4 4.0 Monitoring Summary………………………………………………………………………..5 4.1 Photopoint Monitoring………………………………………………………………...5 4.2 Individual Plant Monitoring…………………………………………………………...8 4.3 Turtle Monitoring……....…………………………………………………………….12 4.4 Aquatic Macroinvertebrate Monitoring…………..………………………………….12 4.5 Stream Temperature Monitoring……………………………………………………..14 4.6 Pollinator Monitoring……………………………………………………..………….17 4.7 Spotted Wing Drosophila Monitoring….…………...…….…………………………19 5.0 Recommendations…………………………………………………………………………..20 5.1 General……………………………………………………………………………….20 5.2 Photopoint Monitoring……………………………………………………………….20 5.3 Individual Plant Monitoring………………………………………………………….20 5.4 Turtle Monitoring…....……………………………………………………………….21 5.5 Aquatic Macroinvertebrate Monitoring………………………………..…………….21 5.6 Stream Temperature Monitoring……………………………………………………..21 5.7 Pollinator Monitoring….…………………….…………………………………….....22 5.8 Spotted Wing Drosophila -

Simarouba Monograph 3/1/04 Simarouba Amara, Glauca
Simarouba Monograph 3/1/04 Simarouba amara, glauca Family: Simaroubaceae Synonyms: Quassia simarouba, Zwingera amara, Picraena officinalis, Simarouba medicinalis Other Common Names: Aceituno, bitterwood, bois blanc, bois amer, bois frene, bois negresse, caixeta, cajú-rana, cedro blanco daguilla, dysentery bark, gavilan, malacacheta, marubá, marupá, negrito, palo blanco, palo amargo, paradise tree, pitomba, robleceillo, simaba. Overview Botanical Description Simarouba is indigenous to the Amazon rainforest and other tropical areas in Mexico, Cuba, Haita, Jamaica and Central America. It grows up to 20 m high and has a trunk 50 to 80 cm in diameter. It produces bright green leaves 20 to 50 cm in length, small white flowers, and small red fruit. Ethnobotanical Uses In traditional herbal medicine systems the bark, wood and leaves of simarouba have been used for their amoebicide, analgesic, anthelmintic, antibacterial, antidysenteric, antimalarial, antimicrobial, astringent, febrifuge, stomachic, sudorific, tonic and vermifuge properties. The traditional use of graviola has been recorded in herbal medicine systems in the following countries: Guiana,1 Belize,2 Brazil,3-6 Cuba,7,8 French Guyana,9 Haiti10 and Peru.11 Summary of Traditional Uses of Simarouba:12 Bark: Anemia, anorexia, bitter,diarrhea, dysentery, dyspepsia, emmenagogue, fever, hemorrhages, internal bleeding, intestinal worms, malaria, skin sores, sores, stomach and bowel disorders, tonic, wounds. Leaf: Astringent, colitis, diarrhea, digestive, dysentery, emmenogogue,intestinal worms, malaria, skin affections. Root: Diarrhea, dysentery, flatulence, intestinal worms, malaria, stomach pain, tonic. Primary Uses Internal A bark tea is primarily used as the first line of defense for amebic dysentery and diarrhea. It’s also used for viruses.13 External The bark has been traditionally used in herbal medicine systems externally for wounds and skin sores.2,7 © Copyrighted 2004. -

Woody-Tissue Respiration for Simarouba Amara and Minquartia Guianensis, Two Tropical Wet Forest Trees with Different Growth Habits
Oecologia (1994) 100:213-220 9Springer-Verlag 1994 Michael G. Ryan 9Robert M. Hubbard Deborah A. Clark 9Robert L. Sanford, Jr. Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits Received: 9 February 1994 / Accepted: 26 July 1994 Abstract We measured CO 2 etflux from stems of two maintenance respiration was 54% of the total CO2 efflux tropical wet forest trees, both found in the canopy, but for Simarouba and 82% for Minquartia. For our sam- with very different growth habits. The species were ple, sapwood volume averaged 23% of stem volume Simarouba amara, a fast-growing species associated when weighted by tree size, or 40% with no size weight- with gaps in old-growth forest and abundant in sec- ing. Using these fractions, and a published estimate of ondary forest, and Minquartia guianensis, a slow-grow- aboveground dry-matter production, we estimate the ing species tolerant of low-light conditions in annual cost of woody tissue respiration for primary old-growth forest. Per unit of bole surface, CO2 efflux forest at La Selva to be 220 or 350 gCm-2year -1, averaged 1.24 gmol m -2 s-1 for Simarouba and depending on the assumed sapwood volume. These 0.83gmolm-2s -1 for Minquartia. C02 elttux was costs are estimated to be less than 13% of the gross highly correlated with annual wood production (r 2= production for the forest. 0.65), but only weakly correlated with stem diameter (r 2 = 0.22). We also partitioned the CO2 efttux into the Key words Woody tissue respiration" Maintenance functional components of construction and mainte- respiration 9Tropical wet forest trees 9Carbon balance nance respiration.