Woody-Tissue Respiration for Simarouba Amara and Minquartia Guianensis, Two Tropical Wet Forest Trees with Different Growth Habits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Agroforestry As a Modern Science
Chapter 2 Evolution of Agroforestry as a Modern Science Jagdish C. Dagar and Vindhya P. Tewari Abstract Agroforestry is as old as agriculture itself. Many of the anecdotal agro- forestry practices, which are time tested and evolved through traditional indigenous knowledge, are still being followed in different agroecological zones. The tradi- tional knowledge and the underlying ecological principles concerning indigenous agroforestry systems around the world have been successfully used in designing the improved systems. Many of them such as improved fallows, homegardens, and park systems have evolved as modern agroforestry systems. During past four decades, agroforestry has come of age and begun to attract the attention of the international scientific community, primarily as a means for sustaining agricultural productivity in marginal lands and solving the second-generation problems such as secondary salinization due to waterlogging and contamination of water resources due to the use of excess nitrogen fertilizers and pesticides. Research efforts have shown that most of the degraded areas including saline, waterlogged, and perturbation ecolo- gies like mine spoils and coastal degraded mangrove areas can be made productive by adopting suitable agroforestry techniques involving highly remunerative compo- nents such as plantation-based farming systems, high-value medicinal and aromatic plants, livestock, fishery, poultry, forest and fruit trees, and vegetables. New con- cepts such as integrated farming systems and urban and peri-urban agroforestry have emerged. Consequently, the knowledge base of agroforestry is being expanded at a rapid pace as illustrated by the increasing number and quality of scientific pub- lications of various forms on different aspects of agroforestry. It is both a challenge and an opportunity to scientific community working in this interdisciplinary field. -

Plants from the Brazilian Traditional Medicine: Species from the Books Of
Revista Brasileira de Farmacognosia 27 (2017) 388–400 ww w.elsevier.com/locate/bjp Original Article Plants from the Brazilian Traditional Medicine: species from the books of the Polish physician Piotr Czerniewicz (Pedro Luiz Napoleão Chernoviz, 1812–1881) a,c d b,c b,c,∗ Letícia M. Ricardo , Juliana de Paula-Souza , Aretha Andrade , Maria G.L. Brandão a Ministério da Saúde, Departamento de Assistência Farmacêutica e Insumos Estratégicos, Esplanada dos Ministérios, Brasília, DF, Brazil b Centro Especializado em Plantas Aromáticas, Medicinais e Tóxicas, Museu de História Natural e Jardim Botânico, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil c Faculdade de Farmácia, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil d Universidade Federal de São João del Rei, Campus Sete Lagoas, Sete Lagoas, MG, Brazil a b s t r a c t a r t i c l e i n f o Article history: The Brazilian flora is very rich in medicinal plants, and much information about the traditional use of Received 1 October 2016 the Brazilian plants is only available from early literature and we are facing a rapid process of loss of Accepted 10 January 2017 biodiversity. To retrieve data about useful plants registered in the books of the Polish physicist P.L.N. Available online 2 March 2017 Chernoviz, who lived in Brazil for 15 years in the 19th century. The aim is to improve our knowledge about Brazilian plants, and to ensure the benefits of sharing it with potential users. Data about Brazilian Keywords: plants were obtained from six editions of the book Formulary and Medical Guide (Formulário e Guia Historical records Médico), published in 1864, 1874, 1888, 1892, 1897 and 1920. -

Tree Height Growth in a Neotropical Rain Forest
Ecology, 82(5), 2001, pp. 1460±1472 q 2001 by the Ecological Society of America GETTING TO THE CANOPY: TREE HEIGHT GROWTH IN A NEOTROPICAL RAIN FOREST DEBORAH A. CLARK1 AND DAVID B. CLARK1 Department of Biology, University of Missouri±St. Louis, 8001 Natural Bridge Road, St. Louis, Missouri 63121-4499, USA Abstract. There is still limited understanding of the processes underlying forest dy- namics in the world's tropical rain forests, ecosystems of disproportionate importance in terms of global biogeochemistry and biodiversity. Particularly poorly documented are the nature and time scale of upward height growth during regeneration by the tree species in these communities. In this study, we assessed long-term height growth through ontogeny for a diverse group of canopy and emergent tree species in a lowland neotropical rain forest (the La Selva Biological Station, northeastern Costa Rica). Species were evaluated based on annual height measurements of large samples of individuals in all postseedling size classes, over a 16-yr period (.11 000 increments). The study species were seven nonpi- oneers (Minquartia guianensis, Lecythis ampla, Hymenolobium mesoamericanum, Sima- rouba amara, Dipteryx panamensis, Balizia elegans, and Hyeronima alchorneoides) and two pioneers (Cecropia obtusifolia and Cecropia insignis). For each species, inherent height growth capacity was estimated as the mean of the ®ve largest annual height increments (from different individuals) in each juvenile size class (from 50 cm tall to 20 cm in diameter). All species showed marked ontogenetic increases in this measure of height growth potential. At all sizes, there were highly signi®cant differences among species in height growth potential. -

How Rare Is Too Rare to Harvest? Management Challenges Posed by Timber Species Occurring at Low Densities in the Brazilian Amazon
Forest Ecology and Management 256 (2008) 1443–1457 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco How rare is too rare to harvest? Management challenges posed by timber species occurring at low densities in the Brazilian Amazon Mark Schulze a,b,c,*, James Grogan b,d, R. Matthew Landis e, Edson Vidal b,f a School of Forest Resources and Conservation, University of Florida, P.O. Box 110760, Gainesville, FL 32611, USA b Instituto do Homem e Meio Ambiente da Amazoˆnia (IMAZON), R. Domingos Marreiros, no. 2020 Bairro Fa´tima, Bele´m, Para´ 66060-160, Brazil c Instituto Floresta Tropical (IFT), Caixa Postal 13077, Bele´m, Para´ 66040-970, Brazil d Yale University School of Forestry & Environmental Studies, 360 Prospect St., New Haven, CT 06511, USA e Department of Biology, Middlebury College, Middlebury, VT 05753, USA f ESALQ/Universidade de Sa˜o Paulo, Piracicaba, Sa˜o Paulo 13.418-900, Brazil ARTICLE INFO ABSTRACT Article history: Tropical forests are characterized by diverse assemblages of plant and animal species compared to Received 11 September 2007 temperate forests. Corollary to this general rule is that most tree species, whether valued for timber or Received in revised form 28 January 2008 not, occur at low densities (<1 adult tree haÀ1) or may be locally rare. In the Brazilian Amazon, many of Accepted 28 February 2008 the most highly valued timber species occur at extremely low densities yet are intensively harvested with Keywords: little regard for impacts on population structures and dynamics. These include big-leaf mahogany Conservation (Swietenia macrophylla), ipeˆ (Tabebuia serratifolia and Tabebuia impetiginosa), jatoba´ (Hymenaea courbaril), Reduced-impact logging and freijo´ cinza (Cordia goeldiana). -

Simarouba Monograph 3/1/04 Simarouba Amara, Glauca
Simarouba Monograph 3/1/04 Simarouba amara, glauca Family: Simaroubaceae Synonyms: Quassia simarouba, Zwingera amara, Picraena officinalis, Simarouba medicinalis Other Common Names: Aceituno, bitterwood, bois blanc, bois amer, bois frene, bois negresse, caixeta, cajú-rana, cedro blanco daguilla, dysentery bark, gavilan, malacacheta, marubá, marupá, negrito, palo blanco, palo amargo, paradise tree, pitomba, robleceillo, simaba. Overview Botanical Description Simarouba is indigenous to the Amazon rainforest and other tropical areas in Mexico, Cuba, Haita, Jamaica and Central America. It grows up to 20 m high and has a trunk 50 to 80 cm in diameter. It produces bright green leaves 20 to 50 cm in length, small white flowers, and small red fruit. Ethnobotanical Uses In traditional herbal medicine systems the bark, wood and leaves of simarouba have been used for their amoebicide, analgesic, anthelmintic, antibacterial, antidysenteric, antimalarial, antimicrobial, astringent, febrifuge, stomachic, sudorific, tonic and vermifuge properties. The traditional use of graviola has been recorded in herbal medicine systems in the following countries: Guiana,1 Belize,2 Brazil,3-6 Cuba,7,8 French Guyana,9 Haiti10 and Peru.11 Summary of Traditional Uses of Simarouba:12 Bark: Anemia, anorexia, bitter,diarrhea, dysentery, dyspepsia, emmenagogue, fever, hemorrhages, internal bleeding, intestinal worms, malaria, skin sores, sores, stomach and bowel disorders, tonic, wounds. Leaf: Astringent, colitis, diarrhea, digestive, dysentery, emmenogogue,intestinal worms, malaria, skin affections. Root: Diarrhea, dysentery, flatulence, intestinal worms, malaria, stomach pain, tonic. Primary Uses Internal A bark tea is primarily used as the first line of defense for amebic dysentery and diarrhea. It’s also used for viruses.13 External The bark has been traditionally used in herbal medicine systems externally for wounds and skin sores.2,7 © Copyrighted 2004. -
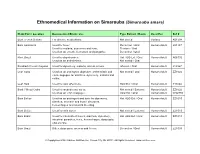
Ethnomedical Information on Simarouba (Simarouba Amara)
Ethnomedical Information on Simarouba (Simarouba amara) Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark French Guiana For diverse medications. Not stated Various K01504 Bark Amazonia Used for fever. Decoction / Oral Human Adult L04137 Used for malaria, dysentery and tonic. Tincture / Oral Used as an emetic, hemostat, and purgative. Decoction / Oral Root Brazil Used to stop diarrhea. Hot H2O Ext / Oral Human Adult A06732 Used as an anthelmintic. Not stated / Oral Rootbark French Guyana Used for dysentery, malaria, and as a tonic. Infusion / Oral Human Adult J12967 Leaf Cuba Used as an astringent, digestive, anthelmintic and Not stated / Oral Human Adult ZZ1022 emmenogogue for diarrhea, dysentery, malaria and colitis. Leaf Haiti Used for skin affections. H2O Ext / Oral Human Adult T13846 Bark / Wood Cuba Used for wounds and sores. Not stated / External Human Adult ZZ1022 Used as an emmenagogue. H2O Ext / Oral Human Adult W02855 Bark Belize Used as an astringent and tonic for dysentery, Hot H2O Ext / Oral Human Adult ZZ1019 diarrhea, stomach and bowel disorders, hemorrhages and internal bleeding. Bark Belize Used for skin sores. Not stated / External Human Adult ZZ1019 Bark Brazil Used for intermittent fevers, diarrhea, dysentery, Hot H2O Ext / Oral Human Adult ZZ1013 intestinal parasites, tonic, hemorrhages, dyspepsia, and anemia. Bark Brazil Bitter, dyspepsia, anemia and fevers. Decoction / Oral Human Adult ZZ1099 © Copyrighted 2004. Raintree Nutrition, Inc. Carson City, NV 89701. All Rights Reserved. www.rain-tree.com Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark + Root Brazil Used for bleeding diarrhea. Decoction / Oral Human Adult ZZ1099 Bark Brazil Used for acute and chronic dysentery, bitter tonic, Infusion / Oral Human Adult ZZ1007 febrifuge, diarrhea, inappetite, and dyspepsia. -

A Preliminary List of the Vascular Plants and Wildlife at the Village Of
A Floristic Evaluation of the Natural Plant Communities and Grounds Occurring at The Key West Botanical Garden, Stock Island, Monroe County, Florida Steven W. Woodmansee [email protected] January 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to CarolAnn Sharkey Key West Botanical Garden 5210 College Road Key West, Florida 33040 and Kate Marks Heritage Preservation 1012 14th Street, NW, Suite 1200 Washington DC 20005 Introduction The Key West Botanical Garden (KWBG) is located at 5210 College Road on Stock Island, Monroe County, Florida. It is a 7.5 acre conservation area, owned by the City of Key West. The KWBG requested that The Institute for Regional Conservation (IRC) conduct a floristic evaluation of its natural areas and grounds and to provide recommendations. Study Design On August 9-10, 2005 an inventory of all vascular plants was conducted at the KWBG. All areas of the KWBG were visited, including the newly acquired property to the south. Special attention was paid toward the remnant natural habitats. A preliminary plant list was established. Plant taxonomy generally follows Wunderlin (1998) and Bailey et al. (1976). Results Five distinct habitats were recorded for the KWBG. Two of which are human altered and are artificial being classified as developed upland and modified wetland. In addition, three natural habitats are found at the KWBG. They are coastal berm (here termed buttonwood hammock), rockland hammock, and tidal swamp habitats. Developed and Modified Habitats Garden and Developed Upland Areas The developed upland portions include the maintained garden areas as well as the cleared parking areas, building edges, and paths. -

The One Hundred Tree Species Prioritized for Planting in the Tropics and Subtropics As Indicated by Database Mining
The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass LIMITED CIRCULATION Correct citation: Kindt R, Dawson IK, Lillesø J-PB, Muchugi A, Pedercini F, Roshetko JM, van Noordwijk M, Graudal L, Jamnadass R. 2021. The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining. Working Paper No. 312. World Agroforestry, Nairobi, Kenya. DOI http://dx.doi.org/10.5716/WP21001.PDF The titles of the Working Paper Series are intended to disseminate provisional results of agroforestry research and practices and to stimulate feedback from the scientific community. Other World Agroforestry publication series include Technical Manuals, Occasional Papers and the Trees for Change Series. Published by World Agroforestry (ICRAF) PO Box 30677, GPO 00100 Nairobi, Kenya Tel: +254(0)20 7224000, via USA +1 650 833 6645 Fax: +254(0)20 7224001, via USA +1 650 833 6646 Email: [email protected] Website: www.worldagroforestry.org © World Agroforestry 2021 Working Paper No. 312 The views expressed in this publication are those of the authors and not necessarily those of World Agroforestry. Articles appearing in this publication series may be quoted or reproduced without charge, provided the source is acknowledged. -

Ailanthus SP.Indd
AILANTHUS Science Page DID YOU KNOW? THE AILANTHUS PLANT .usbg.gov Ailanthus trees grow rapidly, and can reach w Ailanthus can grow in places where 25 meters (80 ft) or more in height. ww almost nothing else All parts of the plant, but will grow. It can especially the flowers, have a strong smell. even grow out of cracks in sidewalks In spring, yellow flowers in polluted cities. appear at the tips of the branches. Male and female flowers are on different trees. male The roots flower UNITEDSTATES BOTANIC GARDEN ORIGINS exude a chemical that Ailanthus is a female hinders the flower native of China. growth of In the US, it was other plants. In summer first planted in and early fall, Philadelphia in The leaves are large clusters of red 1784. It was with pointed leaflets. fruits ripen a popular tree on the female trees. One tree among urban can produce gardeners because thousands of A cluster of its large, fruits each of fruit is year, which are beautiful leaves, rapid growth, and hardiness. Each fruit blown by the 15-30 cm Now the plant is considered a weedy pest 30-60 cm has one (6-12 in) (12-24 in) wind to new seed. long. because it quickly takes over new areas. growing places. CLASSIFYING AILANTHUS FAMILY GENUS SPECIES Simaroubaceae (Quassia family) Ailanthus altissima There are about 150 members in this family. There are about 10 species in “Altissima” is Latin for Most are tropical trees and shrubs. The common name for this genus, all native to Asia “tallest.” Ailanthus grows and Australia. -

Woody and Herbaceous Plants Native to Haiti for Use in Miami-Dade Landscapes1
Woody and Herbaceous Plants Native to Haiti For use in Miami-Dade Landscapes1 Haiti occupies the western one third of the island of Hispaniola with the Dominican Republic the remainder. Of all the islands within the Caribbean basin Hispaniola possesses the most varied flora after that of Cuba. The plants contained in this review have been recorded as native to Haiti, though some may now have been extirpated due in large part to severe deforestation. Less than 1.5% of the country’s original tree-cover remains. Haiti’s future is critically tied to re- forestation; loss of tree cover has been so profound that exotic fast growing trees, rather than native species, are being used to halt soil erosion and lessen the risk of mudslides. For more information concerning Haiti’s ecological plight consult references at the end of this document. For present purposes all of the trees listed below are native to Haiti, which is why non-natives such as mango (the most widely planted tree) and other important trees such as citrus, kassod tree (Senna siamea) and lead tree (Leucanea leucocephala) are not included. The latter two trees are among the fast growing species used for re-forestation. The Smithsonian National Museum of Natural History’s Flora of the West Indies was an invaluable tool in assessing the range of plants native to Haiti. Not surprisingly many of the listed trees and shrubs 1 John McLaughlin Ph.D. U.F./Miami-Dade County Extension Office, Homestead, FL 33030 Page | 1 are found in other parts of the Caribbean with some also native to South Florida. -

Funtumia Elastica (Preuss) Stapf (Apocynaceae)
Journal of Plant Studies; Vol. 8, No. 2; 2019 ISSN 1927-0461 E-ISSN 1927-047X Published by Canadian Center of Science and Education The Main Ecological Characteristics of a Species Introduced to Martinique in a Dynamic of Invasion: Funtumia Elastica (Preuss) Stapf (Apocynaceae) Philippe Joseph1, Kévine Baillard2, Jean-Philippe Claude2, Yelji Abati2, Séverine Ely-Marius2, Yanis Jean-François2, Stéphane Sophie2, Péguy Major2, José Duranty2, Jean Emile Simphor3 & Jean-Valery Marc3 1Professor, UMR ESPACE DEV-BIORECA, University of the French Antilles, French 2Phd Student, UMR ESPACE DEV-BIORECA, University of the French Antilles, French 3Senior Lecturer, UMR ESPACE DEV-BIORECA, University of the French Antilles, French Correspondence: Philippe Joseph, UMR ESPACE DEV-BIORECA, University of the French Antilles, French. Tel: 596-696-206-141. E-mail: [email protected] Received: March 7, 2019 Accepted: April 4, 2019 Online Published: April 30, 2019 doi:10.5539/jps.v8n2p1 URL: https://doi.org/10.5539/jps.v8n2p1 Abstract Introduced species that become invasive alter the structural and functional organisation of the ecosystems of the host territories because of the absence of certain ecological locks. On a global scale, the consequences are very damaging for many key development-related sectors. Martinique, like all the islands of the Caribbean, is not immune to this phenomenon of biological invasion currently linked to greater globalisation. Among the potentially invasive introduced species and in the light of field observations, Funtumia elastica, native to tropical Africa, appears to have functional traits that could make it a species that is dangerous for local floristic diversity. Since no study exists in Martinique on the ecology of this taxon, we have set up a research protocol based on floristic surveys in various stations marked out by transects subdivided into quadrats. -

Redalyc.Physiochemical Analysis and Centesimal Composition Of
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil SALES-CAMPOS, Ceci; Medina ARAUJO, Lidia; Teixeira de Almeida MINHONI, Marli; Nogueira de ANDRADE, Meire Cristina Physiochemical analysis and centesimal composition of Pleurotus ostreatus mushroom grown in residues from the Amazon Ciência e Tecnologia de Alimentos, vol. 31, núm. 2, abril-junio, 2011, pp. 456-461 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395940105027 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ISSN 0101-2061 Ciência e Tecnologia de Alimentos Original Physiochemical analysis and centesimal composition of Pleurotus ostreatus mushroom grown in residues from the Amazon Análise físico-química e composição centecimal do cogumelo Pleurotus ostreatus cultivado em resíduos da Amazônia Ceci SALES-CAMPOS1, Lidia Medina ARAUJO2, Marli Teixeira de Almeida MINHONI3, Meire Cristina Nogueira de ANDRADE3* Abstract The objective of the present work was to evaluate the nutritional composition of mushrooms produced in alternative substrates in agricultural and agro-industrial residues from the Amazon. C, N, pH, moisture, soluble solids, protein, lipids, total fibers, ashes, carbohydrates and energy were determined. Substrates were formulated from Simarouba amara Aubl. and Ochroma piramidale Cav. ex. Lam. Sawdust and from Bactris gasipaes Kunth and Saccharum officinarum stipe. Results showed that the nutritional composition of P. ostreatus varied according to the cultivation substrate and that it can be considered important food due to its nutritional characteristics such as: high protein content; metabolizable carbohydrates and fiber; and low lipids and calories contents.