Funtumia Elastica (Preuss) Stapf (Apocynaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Agroforestry As a Modern Science
Chapter 2 Evolution of Agroforestry as a Modern Science Jagdish C. Dagar and Vindhya P. Tewari Abstract Agroforestry is as old as agriculture itself. Many of the anecdotal agro- forestry practices, which are time tested and evolved through traditional indigenous knowledge, are still being followed in different agroecological zones. The tradi- tional knowledge and the underlying ecological principles concerning indigenous agroforestry systems around the world have been successfully used in designing the improved systems. Many of them such as improved fallows, homegardens, and park systems have evolved as modern agroforestry systems. During past four decades, agroforestry has come of age and begun to attract the attention of the international scientific community, primarily as a means for sustaining agricultural productivity in marginal lands and solving the second-generation problems such as secondary salinization due to waterlogging and contamination of water resources due to the use of excess nitrogen fertilizers and pesticides. Research efforts have shown that most of the degraded areas including saline, waterlogged, and perturbation ecolo- gies like mine spoils and coastal degraded mangrove areas can be made productive by adopting suitable agroforestry techniques involving highly remunerative compo- nents such as plantation-based farming systems, high-value medicinal and aromatic plants, livestock, fishery, poultry, forest and fruit trees, and vegetables. New con- cepts such as integrated farming systems and urban and peri-urban agroforestry have emerged. Consequently, the knowledge base of agroforestry is being expanded at a rapid pace as illustrated by the increasing number and quality of scientific pub- lications of various forms on different aspects of agroforestry. It is both a challenge and an opportunity to scientific community working in this interdisciplinary field. -

A Revision of Farquharia Stapf and Funtumia Stapf (Apocynaceae)
582.937 MEDEDELINGEN LANDBOUWHOGESCHOOL WAGENINGEN • NEDERLAND •81-16(1981) A REVISION OF FARQUHARIA STAPF AND FUNTUMIA STAPF (APOCYNACEAE) H. J. C. ZWETSLOOT Laboratory of Plant Taxonomy and Plant Geography, Agricultural University, Wageningen, The Netherlands Received 21-VII-1981 Date of publication I1-XIM981 H. VEENMAN & ZONEN B.V. - WAGENINGEN - 1981 CONTENTS Introduction History Etymology Geographic distribution . Relationship to other genera Genus/species diagnosis of Farquharia 3 F. elliptica 3 Architecture of Farquharia 9 Nomina nuda referring to Farquharia 9 Phytochemical screening of Farquharia elliptica leaves by T. A. VAN BEEK 9 Genus diagnosis of Funtumia 10 Architecture of Funtumia 11 Discussion of the delimitation of the species of Funtumia 14 Key to the species of Funtumia 16 Species descriptions of Funtumia 16 F. africana 16 F. elastica 25 Somatic chromosome numbers in Funtumia by J. C. ARENDS and F. M. VAN DER LAAN . 32 Phytochemistry of Funtumia by N. G. BISSET 33 Hybrids of Funtumia africana and elastica 34 Statistical keys (Funtumia) 34 References 38 Acknowledgements 41 Index of exsiccatae 42 Register 46 INTRODUCTION The present publication is a monograph of the genera Farquharia and Fun- tumia. It is based on the study of herbarium material and living plants as the author had the opportunity to study flowering and fruiting plants in the field of all species involved. HISTORY Farquharia was described by STAPF in 1912 with a single species F. elliptica. Independantly CHEVALIER proposed the nomina nuda Alafiajasminiflora (1920) and Alqfia mirabilis for the same taxon. HUTCHINSON and DALZIEL (1931) er roneously referred Alafiajasminiflora to the genus Holalafia as they did not notice the clearly apocarpous ovary in the specimen collected by CHEVALIER. -

Medicinal Plant Use and Conservation Practices by Communities in the Togo Plateau Forest Reserve, Ghana
Vol. 12(30), pp. 575-589, 10 December, 2018 DOI: 10.5897/JMPR2018.6686 Article Number: B45C67E59574 ISSN: 1996-0875 Copyright ©2018 Author(s) retain the copyright of this article Journal of Medicinal Plants Research http://www.academicjournals.org/JMPR Full Length Research Paper Medicinal plant use and conservation practices by communities in the Togo Plateau Forest Reserve, Ghana Gilchrist K. Faith Dogor1*, Ruby A. Nyarko1, Alexander K. Anning1 and Alfred 2 Oteng-Yeboah 1Department of Theoretical and Applied Biology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. 2Department of Plant and Environmental Biology, University of Ghana, Accra, Ghana. Received 3 October; Accepted 5 November, 2018 The use of plants for medicine has constituted an important aspect of primary healthcare delivery system of people throughout the world for many centuries. Nonetheless, information on medicinal plants and their conservation are still lacking in some areas, including the Togo Plateau Forest Reserve in Ghana. This paper investigates the ethnobotany of the Togo Plateau Forest Reserve, Ghana, to document the traditional knowledge, uses and conservation of medicinal plants. Information on these plants was sought through a structured questionnaire administered to 384 registered members of the Ghana Federation of Traditional Medicine Practitioners’ Association including certified traditional healers, traditional birth attendants (TBAs), vendors of herbal remedies, managers of herbal medicine centers, and local plant collectors from six communities within the catchment area of the Togo Plateau Forest Reserve. The study recorded 114 medicinal plant species, including 14 herbs, 6 lianas, 21 shrubs and 74 trees that are mostly collected from the wild. The most commonly cited medicinal plant species were Azadirachta indica, Alstonia boonei, Morinda lucidaand Nauclea latifolia, mostly used to treat human conditions such as malaria, jaundice, rheumatism, andcough. -

Antimalarial and Cytotoxic Activities of Pregnene-Type Steroidal Alkaloids from Holarrhena Pubescens Roots
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers: part A Faculty of Science, Medicine and Health 1-1-2017 Antimalarial and cytotoxic activities of pregnene-type steroidal alkaloids from Holarrhena pubescens roots Sarot Cheenpracha University of Phayao Pannakorn Boapun University of Phayao Thunwadee Limtharakul Chiang Mai University Surat Laphookhieo Mae Fah Luang University, [email protected] Stephen G. Pyne University of Wollongong, [email protected] Follow this and additional works at: https://ro.uow.edu.au/smhpapers Part of the Medicine and Health Sciences Commons, and the Social and Behavioral Sciences Commons Recommended Citation Cheenpracha, Sarot; Boapun, Pannakorn; Limtharakul, Thunwadee; Laphookhieo, Surat; and Pyne, Stephen G., "Antimalarial and cytotoxic activities of pregnene-type steroidal alkaloids from Holarrhena pubescens roots" (2017). Faculty of Science, Medicine and Health - Papers: part A. 5110. https://ro.uow.edu.au/smhpapers/5110 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Antimalarial and cytotoxic activities of pregnene-type steroidal alkaloids from Holarrhena pubescens roots Abstract The phytochemical investigation of an alkaloidal extract of Holarrhena pubescens roots led to the isolation and identification of a new pregnene-type alkaloid, mokluangin D (1), together with nine known steroidal alkaloids (2-10). The structure of the new metabolite was determined on the basis of spectroscopic analyses including 1D- and 2D-NMR spectroscopy and mass spectrometry. Compounds 3 and 4 showed potent antimalarial activity against Plasmodium falciparum K1 stain with IC50 values of 1.2 and 2.0 μM, respectively, and showed weak cytotoxic activity against the NCI-H187 cell line with IC50 values of 27.7 and 30.6 μM, respectively. -

Forest Inventory and Analysis National Core Field Guide
National Core Field Guide, Version 5.1 October, 2011 FOREST INVENTORY AND ANALYSIS NATIONAL CORE FIELD GUIDE VOLUME I: FIELD DATA COLLECTION PROCEDURES FOR PHASE 2 PLOTS Version 5.1 National Core Field Guide, Version 5.1 October, 2011 Changes from the Phase 2 Field Guide version 5.0 to version 5.1 Changes documented in change proposals are indicated in bold type. The corresponding proposal name can be seen using the comments feature in the electronic file. • Section 8. Phase 2 (P2) Vegetation Profile (Core Optional). Corrected several figure numbers and figure references in the text. • 8.2. General definitions. NRCS PLANTS database. Changed text from: “USDA, NRCS. 2000. The PLANTS Database (http://plants.usda.gov, 1 January 2000). National Plant Data Center, Baton Rouge, LA 70874-4490 USA. FIA currently uses a stable codeset downloaded in January of 2000.” To: “USDA, NRCS. 2010. The PLANTS Database (http://plants.usda.gov, 1 January 2010). National Plant Data Center, Baton Rouge, LA 70874-4490 USA. FIA currently uses a stable codeset downloaded in January of 2010”. • 8.6.2. SPECIES CODE. Changed the text in the first paragraph from: “Record a code for each sampled vascular plant species found rooted in or overhanging the sampled condition of the subplot at any height. Species codes must be the standardized codes in the Natural Resource Conservation Service (NRCS) PLANTS database (currently January 2000 version). Identification to species only is expected. However, if subspecies information is known, enter the appropriate NRCS code. For graminoids, genus and unknown codes are acceptable, but do not lump species of the same genera or unknown code. -

Tree Height Growth in a Neotropical Rain Forest
Ecology, 82(5), 2001, pp. 1460±1472 q 2001 by the Ecological Society of America GETTING TO THE CANOPY: TREE HEIGHT GROWTH IN A NEOTROPICAL RAIN FOREST DEBORAH A. CLARK1 AND DAVID B. CLARK1 Department of Biology, University of Missouri±St. Louis, 8001 Natural Bridge Road, St. Louis, Missouri 63121-4499, USA Abstract. There is still limited understanding of the processes underlying forest dy- namics in the world's tropical rain forests, ecosystems of disproportionate importance in terms of global biogeochemistry and biodiversity. Particularly poorly documented are the nature and time scale of upward height growth during regeneration by the tree species in these communities. In this study, we assessed long-term height growth through ontogeny for a diverse group of canopy and emergent tree species in a lowland neotropical rain forest (the La Selva Biological Station, northeastern Costa Rica). Species were evaluated based on annual height measurements of large samples of individuals in all postseedling size classes, over a 16-yr period (.11 000 increments). The study species were seven nonpi- oneers (Minquartia guianensis, Lecythis ampla, Hymenolobium mesoamericanum, Sima- rouba amara, Dipteryx panamensis, Balizia elegans, and Hyeronima alchorneoides) and two pioneers (Cecropia obtusifolia and Cecropia insignis). For each species, inherent height growth capacity was estimated as the mean of the ®ve largest annual height increments (from different individuals) in each juvenile size class (from 50 cm tall to 20 cm in diameter). All species showed marked ontogenetic increases in this measure of height growth potential. At all sizes, there were highly signi®cant differences among species in height growth potential. -

How Rare Is Too Rare to Harvest? Management Challenges Posed by Timber Species Occurring at Low Densities in the Brazilian Amazon
Forest Ecology and Management 256 (2008) 1443–1457 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco How rare is too rare to harvest? Management challenges posed by timber species occurring at low densities in the Brazilian Amazon Mark Schulze a,b,c,*, James Grogan b,d, R. Matthew Landis e, Edson Vidal b,f a School of Forest Resources and Conservation, University of Florida, P.O. Box 110760, Gainesville, FL 32611, USA b Instituto do Homem e Meio Ambiente da Amazoˆnia (IMAZON), R. Domingos Marreiros, no. 2020 Bairro Fa´tima, Bele´m, Para´ 66060-160, Brazil c Instituto Floresta Tropical (IFT), Caixa Postal 13077, Bele´m, Para´ 66040-970, Brazil d Yale University School of Forestry & Environmental Studies, 360 Prospect St., New Haven, CT 06511, USA e Department of Biology, Middlebury College, Middlebury, VT 05753, USA f ESALQ/Universidade de Sa˜o Paulo, Piracicaba, Sa˜o Paulo 13.418-900, Brazil ARTICLE INFO ABSTRACT Article history: Tropical forests are characterized by diverse assemblages of plant and animal species compared to Received 11 September 2007 temperate forests. Corollary to this general rule is that most tree species, whether valued for timber or Received in revised form 28 January 2008 not, occur at low densities (<1 adult tree haÀ1) or may be locally rare. In the Brazilian Amazon, many of Accepted 28 February 2008 the most highly valued timber species occur at extremely low densities yet are intensively harvested with Keywords: little regard for impacts on population structures and dynamics. These include big-leaf mahogany Conservation (Swietenia macrophylla), ipeˆ (Tabebuia serratifolia and Tabebuia impetiginosa), jatoba´ (Hymenaea courbaril), Reduced-impact logging and freijo´ cinza (Cordia goeldiana). -

Apocynaceae-Apocynoideae)
THE NERIEAE (APOCYNACEAE-APOCYNOIDEAE) A. J. M. LEEUWENBERG1 ABSTRACT The genera of tribe Nerieae of Apocynaceae are surveyed here and the relationships of the tribe within the family are evaluated. Recent monographic work in the tribe enabled the author to update taxonomie approaches since Pichon (1950) made the last survey. Original observations on the pollen morphology ofth egener a by S.Nilsson ,Swedis h Natural History Museum, Stockholm, are appended to this paper. RÉSUMÉ L'auteur étudie lesgenre s de la tribu desNeriea e desApocynacée s et évalue lesrelation s del a tribu au sein de la famille. Un travail monographique récent sur la tribu a permit à l'auteur de mettre à jour lesapproche s taxonomiques depuis la dernière étude de Pichon (1950). Lesobservation s inédites par S. Nilsson du Muséum d'Histoire Naturelle Suédois à Stockholm sur la morphologie des pollens des genres sontjointe s à cet article. The Apocynaceae have long been divided into it to generic rank and in his arrangement includ two subfamilies, Plumerioideae and Apocynoi- ed Aganosma in the Echitinae. Further, because deae (Echitoideae). Pichon (1947) added a third, of its conspicuous resemblance to Beaumontia, the Cerberioideae, a segregate of Plumerioi it may well be that Amalocalyx (Echiteae— deae—a situation which I have provisionally ac Amalocalycinae, according to Pichon) ought to cepted. These subfamilies were in turn divided be moved to the Nerieae. into tribes and subtribes. Comparative studies Pichon's system is artificial, because he used have shown that the subdivision of the Plume the shape and the indumentum of the area where rioideae is much more natural than that of the the connectives cohere with the head of the pistil Apocynoideae. -

Simarouba Monograph 3/1/04 Simarouba Amara, Glauca
Simarouba Monograph 3/1/04 Simarouba amara, glauca Family: Simaroubaceae Synonyms: Quassia simarouba, Zwingera amara, Picraena officinalis, Simarouba medicinalis Other Common Names: Aceituno, bitterwood, bois blanc, bois amer, bois frene, bois negresse, caixeta, cajú-rana, cedro blanco daguilla, dysentery bark, gavilan, malacacheta, marubá, marupá, negrito, palo blanco, palo amargo, paradise tree, pitomba, robleceillo, simaba. Overview Botanical Description Simarouba is indigenous to the Amazon rainforest and other tropical areas in Mexico, Cuba, Haita, Jamaica and Central America. It grows up to 20 m high and has a trunk 50 to 80 cm in diameter. It produces bright green leaves 20 to 50 cm in length, small white flowers, and small red fruit. Ethnobotanical Uses In traditional herbal medicine systems the bark, wood and leaves of simarouba have been used for their amoebicide, analgesic, anthelmintic, antibacterial, antidysenteric, antimalarial, antimicrobial, astringent, febrifuge, stomachic, sudorific, tonic and vermifuge properties. The traditional use of graviola has been recorded in herbal medicine systems in the following countries: Guiana,1 Belize,2 Brazil,3-6 Cuba,7,8 French Guyana,9 Haiti10 and Peru.11 Summary of Traditional Uses of Simarouba:12 Bark: Anemia, anorexia, bitter,diarrhea, dysentery, dyspepsia, emmenagogue, fever, hemorrhages, internal bleeding, intestinal worms, malaria, skin sores, sores, stomach and bowel disorders, tonic, wounds. Leaf: Astringent, colitis, diarrhea, digestive, dysentery, emmenogogue,intestinal worms, malaria, skin affections. Root: Diarrhea, dysentery, flatulence, intestinal worms, malaria, stomach pain, tonic. Primary Uses Internal A bark tea is primarily used as the first line of defense for amebic dysentery and diarrhea. It’s also used for viruses.13 External The bark has been traditionally used in herbal medicine systems externally for wounds and skin sores.2,7 © Copyrighted 2004. -

Woody-Tissue Respiration for Simarouba Amara and Minquartia Guianensis, Two Tropical Wet Forest Trees with Different Growth Habits
Oecologia (1994) 100:213-220 9Springer-Verlag 1994 Michael G. Ryan 9Robert M. Hubbard Deborah A. Clark 9Robert L. Sanford, Jr. Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits Received: 9 February 1994 / Accepted: 26 July 1994 Abstract We measured CO 2 etflux from stems of two maintenance respiration was 54% of the total CO2 efflux tropical wet forest trees, both found in the canopy, but for Simarouba and 82% for Minquartia. For our sam- with very different growth habits. The species were ple, sapwood volume averaged 23% of stem volume Simarouba amara, a fast-growing species associated when weighted by tree size, or 40% with no size weight- with gaps in old-growth forest and abundant in sec- ing. Using these fractions, and a published estimate of ondary forest, and Minquartia guianensis, a slow-grow- aboveground dry-matter production, we estimate the ing species tolerant of low-light conditions in annual cost of woody tissue respiration for primary old-growth forest. Per unit of bole surface, CO2 efflux forest at La Selva to be 220 or 350 gCm-2year -1, averaged 1.24 gmol m -2 s-1 for Simarouba and depending on the assumed sapwood volume. These 0.83gmolm-2s -1 for Minquartia. C02 elttux was costs are estimated to be less than 13% of the gross highly correlated with annual wood production (r 2= production for the forest. 0.65), but only weakly correlated with stem diameter (r 2 = 0.22). We also partitioned the CO2 efttux into the Key words Woody tissue respiration" Maintenance functional components of construction and mainte- respiration 9Tropical wet forest trees 9Carbon balance nance respiration. -
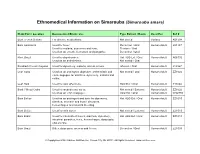
Ethnomedical Information on Simarouba (Simarouba Amara)
Ethnomedical Information on Simarouba (Simarouba amara) Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark French Guiana For diverse medications. Not stated Various K01504 Bark Amazonia Used for fever. Decoction / Oral Human Adult L04137 Used for malaria, dysentery and tonic. Tincture / Oral Used as an emetic, hemostat, and purgative. Decoction / Oral Root Brazil Used to stop diarrhea. Hot H2O Ext / Oral Human Adult A06732 Used as an anthelmintic. Not stated / Oral Rootbark French Guyana Used for dysentery, malaria, and as a tonic. Infusion / Oral Human Adult J12967 Leaf Cuba Used as an astringent, digestive, anthelmintic and Not stated / Oral Human Adult ZZ1022 emmenogogue for diarrhea, dysentery, malaria and colitis. Leaf Haiti Used for skin affections. H2O Ext / Oral Human Adult T13846 Bark / Wood Cuba Used for wounds and sores. Not stated / External Human Adult ZZ1022 Used as an emmenagogue. H2O Ext / Oral Human Adult W02855 Bark Belize Used as an astringent and tonic for dysentery, Hot H2O Ext / Oral Human Adult ZZ1019 diarrhea, stomach and bowel disorders, hemorrhages and internal bleeding. Bark Belize Used for skin sores. Not stated / External Human Adult ZZ1019 Bark Brazil Used for intermittent fevers, diarrhea, dysentery, Hot H2O Ext / Oral Human Adult ZZ1013 intestinal parasites, tonic, hemorrhages, dyspepsia, and anemia. Bark Brazil Bitter, dyspepsia, anemia and fevers. Decoction / Oral Human Adult ZZ1099 © Copyrighted 2004. Raintree Nutrition, Inc. Carson City, NV 89701. All Rights Reserved. www.rain-tree.com Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark + Root Brazil Used for bleeding diarrhea. Decoction / Oral Human Adult ZZ1099 Bark Brazil Used for acute and chronic dysentery, bitter tonic, Infusion / Oral Human Adult ZZ1007 febrifuge, diarrhea, inappetite, and dyspepsia. -

Economically Important Plants Arranged Systematically James P
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 1-2017 Economically Important Plants Arranged Systematically James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: http://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Economically Important Plants Arranged Systematically" (2017). Botanical Studies. 48. http://digitalcommons.humboldt.edu/botany_jps/48 This Economic Botany - Ethnobotany is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. ECONOMICALLY IMPORTANT PLANTS ARRANGED SYSTEMATICALLY Compiled by James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California 30 January 2017 This list began in 1970 as a handout in the Plants and Civilization course that I taught at HSU. It was an updating and expansion of one prepared by Albert F. Hill in his 1952 textbook Economic Botany... and it simply got out of hand. I also thought it would be useful to add a brief description of how the plant is used and what part yields the product. There are a number of more or less encyclopedic references on this subject. The number of plants and the details of their uses is simply overwhelming. In the list below, I have attempted to focus on those plants that are of direct economic importance to us.