Tree Height Growth in a Neotropical Rain Forest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biomimética De Estructuras Vegetales Mejorando La Seguridad En El Ciclismo a Partir De La Olla De Mono
BIOMIMÉTICA DE ESTRUCTURAS VEGETALES MEJORANDO LA SEGURIDAD EN EL CICLISMO A PARTIR DE LA OLLA DE MONO Lucía Paulina Buelvas Álvarez Jenny Alexandra Ramírez Osorio Universidad Pontificia Bolivariana Escuela de Arquitectura y Diseño Facultad de Diseño Industrial Medellín 2014 Línea de morfología experimental CONTENIDO Resumen – Abstract Introducción Planteamiento del proyecto. Tema general del proyecto. Características generales del proyecto de investigación. Problema identifica. Elementos del problema. Hipótesis o pregunta que se formula frente al tema como planteamiento de la investigación. Justificación del proyecto. Validez del proyecto en el contexto de la investigación en diseño industrial. Oportunidades que representa para el desarrollo de nuevos productos o estrategias. Objetivos. Objetivo general. Objetivos específicos. Alcance. Límites y metas temáticas para el proyecto. Límites y metas metodológicas para el proyecto. Marco de referencia. Antecedentes. Estado del arte. Conceptualización de los elementos del problema. Materiales y métodos Material botánico Métodos Caracterización anatómica y morfológica del fruto del árbol Olla de mono Identificación del tipo de materiales del fruto de la Olla de mono Definición del nivel de desempeño funcional estructural del fruto Resultados y discusión Conclusiones Agradecimientos Glosario Bibliografía Biomimética de estructuras vegetales Mejorando la seguridad en el ciclismo a partir de la Olla de mono Lucía Paulina Buelvas Álvarez – Jenny Alexandra Ramírez Osorio Universidad Pontificia Bolivariana, Medellín, Colombia “El éxito parece ser en buena parte cuestión de perseverar después de que otros hayan abandonado.” William Feather Resumen Actualmente el ciclismo es uno de los deportes con mayor nivel de aceptación en Colombia. Para su práctica se requiere del uso de sistemas de protección personal que garanticen la seguridad del deportista, siendo el casco uno de los objetos más importantes de este equipamiento. -

Evolution of Agroforestry As a Modern Science
Chapter 2 Evolution of Agroforestry as a Modern Science Jagdish C. Dagar and Vindhya P. Tewari Abstract Agroforestry is as old as agriculture itself. Many of the anecdotal agro- forestry practices, which are time tested and evolved through traditional indigenous knowledge, are still being followed in different agroecological zones. The tradi- tional knowledge and the underlying ecological principles concerning indigenous agroforestry systems around the world have been successfully used in designing the improved systems. Many of them such as improved fallows, homegardens, and park systems have evolved as modern agroforestry systems. During past four decades, agroforestry has come of age and begun to attract the attention of the international scientific community, primarily as a means for sustaining agricultural productivity in marginal lands and solving the second-generation problems such as secondary salinization due to waterlogging and contamination of water resources due to the use of excess nitrogen fertilizers and pesticides. Research efforts have shown that most of the degraded areas including saline, waterlogged, and perturbation ecolo- gies like mine spoils and coastal degraded mangrove areas can be made productive by adopting suitable agroforestry techniques involving highly remunerative compo- nents such as plantation-based farming systems, high-value medicinal and aromatic plants, livestock, fishery, poultry, forest and fruit trees, and vegetables. New con- cepts such as integrated farming systems and urban and peri-urban agroforestry have emerged. Consequently, the knowledge base of agroforestry is being expanded at a rapid pace as illustrated by the increasing number and quality of scientific pub- lications of various forms on different aspects of agroforestry. It is both a challenge and an opportunity to scientific community working in this interdisciplinary field. -

Diversity and Distributional Patterns of Aroids (Alismatales: Araceae) Along an Elevational Gradient in Darién, Panama
Webbia Journal of Plant Taxonomy and Geography ISSN: 0083-7792 (Print) 2169-4060 (Online) Journal homepage: https://www.tandfonline.com/loi/tweb20 Diversity and distributional patterns of aroids (Alismatales: Araceae) along an elevational gradient in Darién, Panama Orlando O. Ortiz, María S. de Stapf & Thomas B. Croat To cite this article: Orlando O. Ortiz, María S. de Stapf & Thomas B. Croat (2019): Diversity and distributional patterns of aroids (Alismatales: Araceae) along an elevational gradient in Darién, Panama, Webbia, DOI: 10.1080/00837792.2019.1646465 To link to this article: https://doi.org/10.1080/00837792.2019.1646465 View supplementary material Published online: 28 Aug 2019. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tweb20 WEBBIA https://doi.org/10.1080/00837792.2019.1646465 ARTICLE Diversity and distributional patterns of aroids (Alismatales: Araceae) along an elevational gradient in Darién, Panama Orlando O. Ortiz a, María S. de Stapfa,b and Thomas B. Croatc aHerbario PMA, Universidad de Panamá, Estafeta Universitaria, Panama City, Panama; bDepartamento de Botánica, Universidad de Panamá, Estafeta Universitaria, Panama City, Panama; cDepartment of Research (Monographs Section), Missouri Botanical Garden, St. Louis, MO, USA ABSTRACT ARTICLE HISTORY The family Araceae (aroids) represents an ecologically important and diverse group of plants in Received 24 May 2019 Panama, represented by 25 genera, 615 species, of which 277 (45%) are considered endemic. Accepted 18 July 2019 The aim of this study is to analyse the diversity and distributional patterns of aroids along an KEYWORDS fi elevation gradient in the species-rich forests of Darién, Panama. -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

Plants and Gall Hosts of the Tirimbina Biological Reserve
DOI 10.15517/RBT.V67I2SUPL.37233 Artículo Plants and gall hosts of the Tirimbina Biological Reserve, Sarapiqui, Costa Rica: Combining field sampling with herbarium records Plantas y hospederos de agallas de la Reserva Biológica Tirimbina, Sarapiquí, Costa Rica: combinando muestras del campo con registros del herbario Juan Manuel Ley-López1 José González2 Paul E. Hanson3* 1 Departamento Académico, Reserva Biológica Tirimbina. Sarapiquí, Heredia, Costa Rica; [email protected] 2 Independent consultant, Costa Rica; [email protected] 3 Escuela de Biología, Universidad de Costa Rica; San Pedro, 11501-2060 San José, Costa Rica; [email protected] * Correspondence Received 03-X-2018 Corrected 10-I-2018 Accepted 24-I-2019 Abstract There has been an increasing number of inventories of gall-inducing arthropods in the Neotropics. Nonetheless, very few inventories have been carried out in areas where the flora is well documented, and records of galls from herbaria and sites outside the study area have seldom been utilized. In this study we provide a checklist of the native vascular plants of a 345 ha forest reserve in the Caribbean lowlands of Costa Rica and document which of these plants were found to harbor galls. The gall surveys were carried out between November 2013 and December 2016. We also cross-checked our plant list with the previous gall records from elsewhere in the country and searched for galls on herbarium specimens of dicots reported from the reserve. In total, we recorded 143 families and 1174 plant species, of which 401 were hosts of galls. Plant hosts of galls were found in the following non-mutually exclusive categories: 209 in our field sampling, 257 from previous records, and 158 in herbarium specimens. -

Plants from the Brazilian Traditional Medicine: Species from the Books Of
Revista Brasileira de Farmacognosia 27 (2017) 388–400 ww w.elsevier.com/locate/bjp Original Article Plants from the Brazilian Traditional Medicine: species from the books of the Polish physician Piotr Czerniewicz (Pedro Luiz Napoleão Chernoviz, 1812–1881) a,c d b,c b,c,∗ Letícia M. Ricardo , Juliana de Paula-Souza , Aretha Andrade , Maria G.L. Brandão a Ministério da Saúde, Departamento de Assistência Farmacêutica e Insumos Estratégicos, Esplanada dos Ministérios, Brasília, DF, Brazil b Centro Especializado em Plantas Aromáticas, Medicinais e Tóxicas, Museu de História Natural e Jardim Botânico, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil c Faculdade de Farmácia, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil d Universidade Federal de São João del Rei, Campus Sete Lagoas, Sete Lagoas, MG, Brazil a b s t r a c t a r t i c l e i n f o Article history: The Brazilian flora is very rich in medicinal plants, and much information about the traditional use of Received 1 October 2016 the Brazilian plants is only available from early literature and we are facing a rapid process of loss of Accepted 10 January 2017 biodiversity. To retrieve data about useful plants registered in the books of the Polish physicist P.L.N. Available online 2 March 2017 Chernoviz, who lived in Brazil for 15 years in the 19th century. The aim is to improve our knowledge about Brazilian plants, and to ensure the benefits of sharing it with potential users. Data about Brazilian Keywords: plants were obtained from six editions of the book Formulary and Medical Guide (Formulário e Guia Historical records Médico), published in 1864, 1874, 1888, 1892, 1897 and 1920. -

How Rare Is Too Rare to Harvest? Management Challenges Posed by Timber Species Occurring at Low Densities in the Brazilian Amazon
Forest Ecology and Management 256 (2008) 1443–1457 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco How rare is too rare to harvest? Management challenges posed by timber species occurring at low densities in the Brazilian Amazon Mark Schulze a,b,c,*, James Grogan b,d, R. Matthew Landis e, Edson Vidal b,f a School of Forest Resources and Conservation, University of Florida, P.O. Box 110760, Gainesville, FL 32611, USA b Instituto do Homem e Meio Ambiente da Amazoˆnia (IMAZON), R. Domingos Marreiros, no. 2020 Bairro Fa´tima, Bele´m, Para´ 66060-160, Brazil c Instituto Floresta Tropical (IFT), Caixa Postal 13077, Bele´m, Para´ 66040-970, Brazil d Yale University School of Forestry & Environmental Studies, 360 Prospect St., New Haven, CT 06511, USA e Department of Biology, Middlebury College, Middlebury, VT 05753, USA f ESALQ/Universidade de Sa˜o Paulo, Piracicaba, Sa˜o Paulo 13.418-900, Brazil ARTICLE INFO ABSTRACT Article history: Tropical forests are characterized by diverse assemblages of plant and animal species compared to Received 11 September 2007 temperate forests. Corollary to this general rule is that most tree species, whether valued for timber or Received in revised form 28 January 2008 not, occur at low densities (<1 adult tree haÀ1) or may be locally rare. In the Brazilian Amazon, many of Accepted 28 February 2008 the most highly valued timber species occur at extremely low densities yet are intensively harvested with Keywords: little regard for impacts on population structures and dynamics. These include big-leaf mahogany Conservation (Swietenia macrophylla), ipeˆ (Tabebuia serratifolia and Tabebuia impetiginosa), jatoba´ (Hymenaea courbaril), Reduced-impact logging and freijo´ cinza (Cordia goeldiana). -

Simarouba Monograph 3/1/04 Simarouba Amara, Glauca
Simarouba Monograph 3/1/04 Simarouba amara, glauca Family: Simaroubaceae Synonyms: Quassia simarouba, Zwingera amara, Picraena officinalis, Simarouba medicinalis Other Common Names: Aceituno, bitterwood, bois blanc, bois amer, bois frene, bois negresse, caixeta, cajú-rana, cedro blanco daguilla, dysentery bark, gavilan, malacacheta, marubá, marupá, negrito, palo blanco, palo amargo, paradise tree, pitomba, robleceillo, simaba. Overview Botanical Description Simarouba is indigenous to the Amazon rainforest and other tropical areas in Mexico, Cuba, Haita, Jamaica and Central America. It grows up to 20 m high and has a trunk 50 to 80 cm in diameter. It produces bright green leaves 20 to 50 cm in length, small white flowers, and small red fruit. Ethnobotanical Uses In traditional herbal medicine systems the bark, wood and leaves of simarouba have been used for their amoebicide, analgesic, anthelmintic, antibacterial, antidysenteric, antimalarial, antimicrobial, astringent, febrifuge, stomachic, sudorific, tonic and vermifuge properties. The traditional use of graviola has been recorded in herbal medicine systems in the following countries: Guiana,1 Belize,2 Brazil,3-6 Cuba,7,8 French Guyana,9 Haiti10 and Peru.11 Summary of Traditional Uses of Simarouba:12 Bark: Anemia, anorexia, bitter,diarrhea, dysentery, dyspepsia, emmenagogue, fever, hemorrhages, internal bleeding, intestinal worms, malaria, skin sores, sores, stomach and bowel disorders, tonic, wounds. Leaf: Astringent, colitis, diarrhea, digestive, dysentery, emmenogogue,intestinal worms, malaria, skin affections. Root: Diarrhea, dysentery, flatulence, intestinal worms, malaria, stomach pain, tonic. Primary Uses Internal A bark tea is primarily used as the first line of defense for amebic dysentery and diarrhea. It’s also used for viruses.13 External The bark has been traditionally used in herbal medicine systems externally for wounds and skin sores.2,7 © Copyrighted 2004. -

Woody-Tissue Respiration for Simarouba Amara and Minquartia Guianensis, Two Tropical Wet Forest Trees with Different Growth Habits
Oecologia (1994) 100:213-220 9Springer-Verlag 1994 Michael G. Ryan 9Robert M. Hubbard Deborah A. Clark 9Robert L. Sanford, Jr. Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits Received: 9 February 1994 / Accepted: 26 July 1994 Abstract We measured CO 2 etflux from stems of two maintenance respiration was 54% of the total CO2 efflux tropical wet forest trees, both found in the canopy, but for Simarouba and 82% for Minquartia. For our sam- with very different growth habits. The species were ple, sapwood volume averaged 23% of stem volume Simarouba amara, a fast-growing species associated when weighted by tree size, or 40% with no size weight- with gaps in old-growth forest and abundant in sec- ing. Using these fractions, and a published estimate of ondary forest, and Minquartia guianensis, a slow-grow- aboveground dry-matter production, we estimate the ing species tolerant of low-light conditions in annual cost of woody tissue respiration for primary old-growth forest. Per unit of bole surface, CO2 efflux forest at La Selva to be 220 or 350 gCm-2year -1, averaged 1.24 gmol m -2 s-1 for Simarouba and depending on the assumed sapwood volume. These 0.83gmolm-2s -1 for Minquartia. C02 elttux was costs are estimated to be less than 13% of the gross highly correlated with annual wood production (r 2= production for the forest. 0.65), but only weakly correlated with stem diameter (r 2 = 0.22). We also partitioned the CO2 efttux into the Key words Woody tissue respiration" Maintenance functional components of construction and mainte- respiration 9Tropical wet forest trees 9Carbon balance nance respiration. -
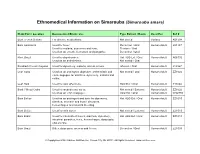
Ethnomedical Information on Simarouba (Simarouba Amara)
Ethnomedical Information on Simarouba (Simarouba amara) Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark French Guiana For diverse medications. Not stated Various K01504 Bark Amazonia Used for fever. Decoction / Oral Human Adult L04137 Used for malaria, dysentery and tonic. Tincture / Oral Used as an emetic, hemostat, and purgative. Decoction / Oral Root Brazil Used to stop diarrhea. Hot H2O Ext / Oral Human Adult A06732 Used as an anthelmintic. Not stated / Oral Rootbark French Guyana Used for dysentery, malaria, and as a tonic. Infusion / Oral Human Adult J12967 Leaf Cuba Used as an astringent, digestive, anthelmintic and Not stated / Oral Human Adult ZZ1022 emmenogogue for diarrhea, dysentery, malaria and colitis. Leaf Haiti Used for skin affections. H2O Ext / Oral Human Adult T13846 Bark / Wood Cuba Used for wounds and sores. Not stated / External Human Adult ZZ1022 Used as an emmenagogue. H2O Ext / Oral Human Adult W02855 Bark Belize Used as an astringent and tonic for dysentery, Hot H2O Ext / Oral Human Adult ZZ1019 diarrhea, stomach and bowel disorders, hemorrhages and internal bleeding. Bark Belize Used for skin sores. Not stated / External Human Adult ZZ1019 Bark Brazil Used for intermittent fevers, diarrhea, dysentery, Hot H2O Ext / Oral Human Adult ZZ1013 intestinal parasites, tonic, hemorrhages, dyspepsia, and anemia. Bark Brazil Bitter, dyspepsia, anemia and fevers. Decoction / Oral Human Adult ZZ1099 © Copyrighted 2004. Raintree Nutrition, Inc. Carson City, NV 89701. All Rights Reserved. www.rain-tree.com Plant Part / Location Documented Ethnic Use Type Extract / Route Used For Ref # Bark + Root Brazil Used for bleeding diarrhea. Decoction / Oral Human Adult ZZ1099 Bark Brazil Used for acute and chronic dysentery, bitter tonic, Infusion / Oral Human Adult ZZ1007 febrifuge, diarrhea, inappetite, and dyspepsia. -

A Preliminary List of the Vascular Plants and Wildlife at the Village Of
A Floristic Evaluation of the Natural Plant Communities and Grounds Occurring at The Key West Botanical Garden, Stock Island, Monroe County, Florida Steven W. Woodmansee [email protected] January 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to CarolAnn Sharkey Key West Botanical Garden 5210 College Road Key West, Florida 33040 and Kate Marks Heritage Preservation 1012 14th Street, NW, Suite 1200 Washington DC 20005 Introduction The Key West Botanical Garden (KWBG) is located at 5210 College Road on Stock Island, Monroe County, Florida. It is a 7.5 acre conservation area, owned by the City of Key West. The KWBG requested that The Institute for Regional Conservation (IRC) conduct a floristic evaluation of its natural areas and grounds and to provide recommendations. Study Design On August 9-10, 2005 an inventory of all vascular plants was conducted at the KWBG. All areas of the KWBG were visited, including the newly acquired property to the south. Special attention was paid toward the remnant natural habitats. A preliminary plant list was established. Plant taxonomy generally follows Wunderlin (1998) and Bailey et al. (1976). Results Five distinct habitats were recorded for the KWBG. Two of which are human altered and are artificial being classified as developed upland and modified wetland. In addition, three natural habitats are found at the KWBG. They are coastal berm (here termed buttonwood hammock), rockland hammock, and tidal swamp habitats. Developed and Modified Habitats Garden and Developed Upland Areas The developed upland portions include the maintained garden areas as well as the cleared parking areas, building edges, and paths. -

The One Hundred Tree Species Prioritized for Planting in the Tropics and Subtropics As Indicated by Database Mining
The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass LIMITED CIRCULATION Correct citation: Kindt R, Dawson IK, Lillesø J-PB, Muchugi A, Pedercini F, Roshetko JM, van Noordwijk M, Graudal L, Jamnadass R. 2021. The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining. Working Paper No. 312. World Agroforestry, Nairobi, Kenya. DOI http://dx.doi.org/10.5716/WP21001.PDF The titles of the Working Paper Series are intended to disseminate provisional results of agroforestry research and practices and to stimulate feedback from the scientific community. Other World Agroforestry publication series include Technical Manuals, Occasional Papers and the Trees for Change Series. Published by World Agroforestry (ICRAF) PO Box 30677, GPO 00100 Nairobi, Kenya Tel: +254(0)20 7224000, via USA +1 650 833 6645 Fax: +254(0)20 7224001, via USA +1 650 833 6646 Email: [email protected] Website: www.worldagroforestry.org © World Agroforestry 2021 Working Paper No. 312 The views expressed in this publication are those of the authors and not necessarily those of World Agroforestry. Articles appearing in this publication series may be quoted or reproduced without charge, provided the source is acknowledged.