Effect of Precious Metals on NO Reduction by CO in Oxidative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Process for Purification of Ethylene Compound Having Fluorine-Containing Organic Group
Office europeen des brevets (fi) Publication number : 0 506 374 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 92302586.0 @ Int. CI.5: C07C 17/38, C07C 21/18 (g) Date of filing : 25.03.92 (30) Priority : 26.03.91 JP 86090/91 (72) Inventor : Kishita, Hirofumi 3-19-1, Isobe, Annaka-shi Gunma-ken (JP) (43) Date of publication of application : Inventor : Sato, Shinichi 30.09.92 Bulletin 92/40 3-5-5, Isobe, Annaka-shi Gunma-ken (JP) Inventor : Fujii, Hideki (84) Designated Contracting States : 3-12-37, Isobe, Annaka-shi DE FR GB Gunma-ken (JP) Inventor : Matsuda, Takashi 791 ~4 YdndSG Anri3k3~shi @ Applicant : SHIN-ETSU CHEMICAL CO., LTD. Gunma-ken (JP) 6-1, Ohtemachi 2-chome Chiyoda-ku Tokyo (JP) @) Representative : Votier, Sidney David et al CARPMAELS & RANSFORD 43, Bloomsbury Square London WC1A 2RA (GB) (54) Process for purification of ethylene compound having fluorine-containing organic group. (57) A process for purifying an ethylene compound having a fluorine-containing organic group (fluorine- containing ethylene compound) by mixing the fluorine-containing ethylene compound with an alkali metal or alkaline earth metal reducing agent, and subjecting the resulting mixture to irradiation with ultraviolet radiation, followed by washing with water. The purification process ensures effective removal of iodides which are sources of molecular iodine, from the fluorine-containing ethylene compound. < h- CO CO o 10 o Q_ LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 506 374 A1 BACKGROUND OF THE INVENTION 1. Field of the Invention 5 The present invention relates to a process for purifying an ethylene compound having a fluorine-containing organic group, and more particularly to a purification process by which iodine contained as impurity in an ethylene compound having a fluorine-containing organic group can be removed effectively. -
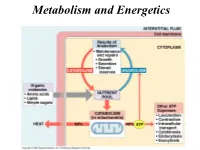
Metabolism and Energetics Oxidation of Carbon Atoms of Glucose Is the Major Source of Energy in Aerobic Metabolism
Metabolism and Energetics Oxidation of carbon atoms of glucose is the major source of energy in aerobic metabolism C6H1206 + 6O2 yields 6 CO2 + H20 + energy Energy released ΔG = - 686 kcal/mol Glucose oxidation requires over 25 discrete steps, with production of 36 ATP. Energy Transformations The mitochondrial synthesis of ATP is not stochiometric. Electron–motive force Proton-motive force Phosphoryl-transfer potential in the form of ATP. Substrate level phosphorylation The formation of ATP by substrate-level phosphorylation ADP ATP P CH O P CH2 O P 2 is used to represent HC OH HC OH a phosphate ester: phosphoglycerate CH OH OH CH2 O P kinase 2 P O bisphosphoglycerate 3-phosphoglycerate OH ADP ATP CH2 CH2 CH3 C O P C OH C O pyruvate kinase non-enzymic COOH COOH COOH phosphoenolpyruvate enolpyruvate pyruvate Why ATP? The reaction of ATP hydrolysis is very favorable ΔGo = - 30.5 kJ/mol = - 7.3 kCal/mol 1. Charge separation of closely packed phosphate groups provides electrostatic relief. Mg2+ 2. Inorganic Pi, the product of the reaction, is immediately resonance-stabilized (electron density spreads equally to all oxygens). 3. ADP immediately ionizes giving H+ into a low [H+] environment (pH~7). 4. Both Pi and ADP are more favorably solvated by water than one ATP molecule. 5. ATP is water soluble. The total body content of ATP and ADP is under 350 mmol – about 10 g, BUT … the amount of ATP synthesized and used each day is about 150 mol – about 110 kg. ATP Production - stage 1 - Glycolysis Glycolysis When rapid production of ATP is needed. -

Reductions and Reducing Agents
REDUCTIONS AND REDUCING AGENTS 1 Reductions and Reducing Agents • Basic definition of reduction: Addition of hydrogen or removal of oxygen • Addition of electrons 9:45 AM 2 Reducible Functional Groups 9:45 AM 3 Categories of Common Reducing Agents 9:45 AM 4 Relative Reactivity of Nucleophiles at the Reducible Functional Groups In the absence of any secondary interactions, the carbonyl compounds exhibit the following order of reactivity at the carbonyl This order may however be reversed in the presence of unique secondary interactions inherent in the molecule; interactions that may 9:45 AM be activated by some property of the reacting partner 5 Common Reducing Agents (Borohydrides) Reduction of Amides to Amines 9:45 AM 6 Common Reducing Agents (Borohydrides) Reduction of Carboxylic Acids to Primary Alcohols O 3 R CO2H + BH3 R O B + 3 H 3 2 Acyloxyborane 9:45 AM 7 Common Reducing Agents (Sodium Borohydride) The reductions with NaBH4 are commonly carried out in EtOH (Serving as a protic solvent) Note that nucleophilic attack occurs from the least hindered face of the 8 carbonyl Common Reducing Agents (Lithium Borohydride) The reductions with LiBH4 are commonly carried out in THF or ether Note that nucleophilic attack occurs from the least hindered face of the 9:45 AM 9 carbonyl. Common Reducing Agents (Borohydrides) The Influence of Metal Cations on Reactivity As a result of the differences in reactivity between sodium borohydride and lithium borohydride, chemoselectivity of reduction can be achieved by a judicious choice of reducing agent. 9:45 AM 10 Common Reducing Agents (Sodium Cyanoborohydride) 9:45 AM 11 Common Reducing Agents (Reductive Amination with Sodium Cyanoborohydride) 9:45 AM 12 Lithium Aluminium Hydride Lithium aluminiumhydride reacts the same way as lithium borohydride. -

Chapter 20 Electrochemistry
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method of half-reactions. Sketch a voltaic cell and identify its cathode, anode, and the directions in which electrons and ions move. o Calculate standard emfs (cell potentials), E cell, from standard reduction potentials. Use reduction potentials to predict whether a redox reaction is spontaneous. o o Relate E cell to DG and equilibrium constants. Calculate emf under nonstandard conditions. Identify the components of common batteries. Describe the construction of a lithium-ion battery and explain how it works. Describe the construction of a fuel cell and explain how it generates electrical energy. Explain how corrosion occurs and how it is prevented by cathodic protection. Describe the reactions in electrolytic cells. Relate the amounts of products and reactants in redox reactions to electrical charge. Electrochemistry Electrochemistry is the study of the relationships between electricity and chemical reactions. • It includes the study of both spontaneous and nonspontaneous processes. 1 Redox reactions: assigning oxidation numbers Oxidation numbers help keep track of what species loses electrons and what species gains them. • An element is oxidized when the oxidation number increases • An element is reduced when the oxidation number decreases • an oxidizing agent causes another element to be oxidized • a reducing agent causes another element to be reduced. Assigning oxidation numbers (sect. 4.4) 1. Elemental form, each atom has ox. # = 0. Zn O2 O3 I2 S8 P4 2. Simple ions, = charge on ion. -1 for Cl-, +2 for Mg2+ 3. -

Ch. 21.1 Redox Reactions and Electrochemical Cells
Pre-Health Post-Baccalaureate Program Study Guide and Practice Problems Course: CHM2046 Textbook Chapter: 21.1 (Silberberg 6e) Topics Covered: Redox Reactions and Electrochemical Cells Created by Isaac Loy 1. Review Understanding this chapter’s material will depend on an in- depth understanding of redox reactions, which were first covered last semester in ch. 4. We will review redox reactions in this study guide, but it would be wise to review ch. 4 if you are having difficulty with this material. Redox reactions will also be incredibly important moving forward into organic chemistry and biochemistry. 2. Oxidation-Reduction Reactions The mnemonic that you will come back to time and time again for this topic is: “LEO the lion says GER” Where “LEO” stands for Loss of Electrons = Oxidation And “GER” stands for Gain of Electrons = Reduction The oxidizing agent (the substance that is being reduced) pulls electrons from the substance that is being oxidized. The reducing agent (the substance that is being oxidized) gives electrons to the substance that is being reduced. Oxidation and reduction are simultaneous processes. In order for a redox reaction to take place, one substance must be oxidized and the other must be reduced. When working with oxidation numbers to solve problems, the substance being oxidized (LEO -> loss of electrons) becomes more positive. Likewise, the substance being reduced (GER -> gaining electrons) becomes more negative. 3. Using Half-Reactions to Solve Redox Problems Follow the steps below to create half-reactions. It is crucial that you follow the steps in order! A. Split up the overall reaction into two half-reactions, where the species of one reaction is being oxidized, and the species of the other reaction is being reduced. -

Gen Chem II Jasperse Ch. 19 Electrochemistry 1
Gen Chem II Jasperse Ch. 19 Electrochemistry 1 Chapter 19 Electrochemistry Math Summary Relating Standard Cell Potential to Standard Half Cell Potentials Eºcell=Eºoxidation + Eºreduction (standard conditions assume 1.0 M concentrations) Relating Half Cell Potentials when Written in Opposite Directions Eºox = -Eºred for half reactions written in opposite directions Relating Standard Cell Potentials to ∆G ∆Gº = -nFE˚cell (to give answer in kJ, use F = 96.485) F = 96,500 C/mol n=number of electrons transferred Relating Actual Cell Potential to Standard Cell Potential when Concentrations aren't 1.0-M Ecell = Eºcell -[0.0592/n] log Q (Q = ratio of actual concentrations) Relating Standard Cell Potential to Equilibrium Constant log K = nEº/0.0592 Relating Actual Cell Potential to Actual Concentrations in Concentration Cells Ecell = -[0.0592/n] log Q for concentration cells, where anode and cathode differ only in concentration, but otherwise have same ions Relating # of Moles of Electrons Transferred as a Function of Time and Current in Electrolysis 1 mol e- = 96,500 C moles of electrons = [current (A)•time (sec)]/96,500 for electrolysis, moles, current, and time are related. rearranged: time (sec)=(moles of electrons)(96500)/current (in A) Note: 3600 sec/hour so time (hours)=(moles of electrons)(26.8)/current (in A) Electrochemistry-Related Units C = Coulomb = quantity of electrical charge = 6.24 • 1018 electrons • 1 mole of electrons = 96,500 C A = amp = rate of charge flow per time = C/sec V = volt = electrical power/force/strength = J/C 96,500C 96.5 kJ F = Faraday = = mole e− mole e− •V € € Gen Chem II Jasperse Ch. -

Evolution of the First Metabolic Cycles
Proc. Natl. Acad. Sci. USA Vol. 87, pp. 200-204, January 1990 Evolution Evolution of the first metabolic cycles (chemoautotrophy/reductive citric acid cycle/origin of life/pyrite) GUNTER WACHTERSHAUSER 8000 Munich 2, Tal 29, Federal Republic of Germany Communicated by Karl Popper, October 12, 1989 (received for review February 28, 1989) ABSTRACT There are two alternatives concerning the genobacter thermophilus (13), and Desulfobacter hydro- origin of life: the origin may be heterotrophic or autotrophic. genophilus (14) and also in the sulfur-associated archaebac- The central problem within the theory of an autotrophic origin teria Thermoproteus neutrophilus (15) and, partly demon- is the first process of carbon fixation. I here propose the strated, in Sulfolobus brierleyi (16). As suggested by Kandler hypothesis that this process is an autocatalytic cycle that can be and Stetter (16) and previously by Hartmann (17), it may be retrodictively constructed from the extant reductive citric acid considered to be of great antiquity and the evolutionary cycle by replacing thioesters by thioacids and by assuming that precursor ofthe oxidative Krebs cycle. It is here conjectured the required reducing power is obtained from the oxidative to be the extant candidate for the reconstruction of the formation of pyrite (FeS2). This archaic cycle is strictly archaic autocatalytic cycle of carbon fixation. chemoautotrophic: photoautotrophy is not required. The cycle The presently accepted form of the extant reductive citric is catalytic for pyrite formation and autocatalytic for its own acid cycle is shown in Fig. 1 in a somewhat unusual repre- multiplication. It is a consequence of this hypothesis that the sentation, twisted in an 8. -

20 More About Oxidation–Reduction Reactions
More About 20 Oxidation–Reduction Reactions OOC n important group of organic reactions consists of those that O A involve the transfer of electrons C from one molecule to another. Organic chemists H OH use these reactions—called oxidation–reduction reactions or redox reactions—to synthesize a large O variety of compounds. Redox reactions are also important C in biological systems because many of these reactions produce HH energy. You have seen a number of oxidation and reduction reactions in other chapters, but discussing them as a group will give you the opportunity to CH3OH compare them. In an oxidation–reduction reaction, one compound loses electrons and one com- pound gains electrons. The compound that loses electrons is oxidized, and the one that gains electrons is reduced. One way to remember the difference between oxidation and reduction is with the phrase “LEO the lion says GER”: Loss of Electrons is Oxi- dation; Gain of Electrons is Reduction. The following is an example of an oxidation–reduction reaction involving inorganic reagents: Cu+ + Fe3+ ¡ Cu2+ + Fe2+ In this reaction,Cu+ loses an electron, so Cu+ is oxidized. Fe3+ gains an electron, so Fe3+ is reduced. The reaction demonstrates two important points about oxidation– reduction reactions. First, oxidation is always coupled with reduction. In other words, a compound cannot gain electrons (be reduced) unless another compound in the reaction simultaneously loses electrons (is oxidized). Second, the compound that is oxidized (Cu+) is called the reducing agent because it loses the electrons that are used to reduce the other compound (Fe3+). Similarly, the compound that is reduced (Fe3+) is called the oxidizing agent because it gains the electrons given up by the other compound (Cu+) when it is oxidized. -

Chemical Redox Agents for Organometallic Chemistry
Chem. Rev. 1996, 96, 877−910 877 Chemical Redox Agents for Organometallic Chemistry Neil G. Connelly*,† and William E. Geiger*,‡ School of Chemistry, University of Bristol, U.K., and Department of Chemistry, University of Vermont, Burlington, Vermont 05405-0125 Received October 3, 1995 (Revised Manuscript Received January 9, 1996) Contents I. Introduction 877 A. Scope of the Review 877 B. Benefits of Redox Agents: Comparison with 878 Electrochemical Methods 1. Advantages of Chemical Redox Agents 878 2. Disadvantages of Chemical Redox Agents 879 C. Potentials in Nonaqueous Solvents 879 D. Reversible vs Irreversible ET Reagents 879 E. Categorization of Reagent Strength 881 II. Oxidants 881 A. Inorganic 881 1. Metal and Metal Complex Oxidants 881 2. Main Group Oxidants 887 B. Organic 891 The authors (Bill Geiger, left; Neil Connelly, right) have been at the forefront of organometallic electrochemistry for more than 20 years and have had 1. Radical Cations 891 a long-standing and fruitful collaboration. 2. Carbocations 893 3. Cyanocarbons and Related Electron-Rich 894 Neil Connelly took his B.Sc. (1966) and Ph.D. (1969, under the direction Compounds of Jon McCleverty) degrees at the University of Sheffield, U.K. Post- 4. Quinones 895 doctoral work at the Universities of Wisconsin (with Lawrence F. Dahl) 5. Other Organic Oxidants 896 and Cambridge (with Brian Johnson and Jack Lewis) was followed by an appointment at the University of Bristol (Lectureship, 1971; D.Sc. degree, III. Reductants 896 1973; Readership 1975). His research interests are centered on synthetic A. Inorganic 896 and structural studies of redox-active organometallic and coordination 1. -

Redox Reactions Is
Chapter 10 OXIDATION-REDUCTION REACTIONS Oxidation – reduction reactions are those involving the transfer of electrons from one substance to another (no bonding formed or broken). Example: Fe 3+ + e- çè Fe 2+ Protons (H+) are often involved in these reactions also. Another example of redox reactions is: - + H2O2 + 2e + 2H 2H2O Rules for the assigning of oxidation numbers 1. All species in their elemental form are given the oxidatio435n number of zero. 2. All monoatomic ions have the same oxidation number as the charge on the ion. e.g. Mg 2+ has the oxidation number of +2. 3. All combined hydrogen has an oxidation number of +1 (except metal hydrides where its oxidation number is -1). 4. All combined oxygen has an oxidation number of -2 (except peroxides where the oxidation number is -1). 5. In polyatomic species, the sum of the oxidation numbers of the element in the ion equals the charge on that species (we can use this to find the oxidation number of elements in polyatomic species). A. Source of electrons in soils. Electrons dTPSSo not flow around by themselves but they m ust be provided by some sources . In soils, the main source of electrons is carbon atoms of organic matter because carbon has a wide range of oxidation states. Example: CH4 + 2O2 2H2O + CO2 Ox. # (-4) Ox. # (+4) - The reaction (CH4 ® CO2) releases 8e Other common sources of e – are nitrogen and sulfur atoms because they can also have several oxidation states. The availability of electrons usually controls the oxidation/reduction reactions and this availability is expressed as redox potentials. -

II Reduction Reactions
II Reduction Reactions Objectives By the end of this section you will: 1) be able to exploit the differences in reactivity of various reducing agents (hydride vs neutral reductants) in chemoselective reductions and be able to provide a mechanistic rationale to account for their differing reactivities; 2) be able to use the inherent chirality in a substrate to control the outcome of a reduction of proximal ketones to generate selectively syn and anti 1,3- and 1,2-diols; 3) be able to rationalise the outcome of these diastereoselective reactions using T.S. diagrams; 4) have gained an appreciation of the versatility of transition metals in reduction reactions; 5) have gained an appreciation of the synthetic utility of dissolving metal reductions; 6) be able to use radical chemistry for deoxygenation and reduction of halides. II.A Reduction of Carboxylic Acid Derivatives and Related Functionality OR' H ROH RO RO carboxylic acid aldehyde primary alcohol derivatives R N RNH2 RNO2 Issues of Reactivity and Selectivity Similar issues of selectivity and reactivity to those we encountered in the case of oxidation reactions also arise in reduction reactions. 1. Chemoselectivity. Many different functional groups can be reduced in a variety of ways. We often need to selectively reduce one functional group whilst leaving others intact (remember year 1 practical!). NaBH4 Sn, HCl OH O O O2N O2N H2N Chemoselective reductions from a practical in CHM1C3 2. In the case of carboxylic acid derivatives there are two possible reduction products: an aldehyde and an alcohol. Ideally we need methods for selectively accessing either product. -

Voltaic Cells
Electrochemistry & Redox Voltaic Cells A voltaic electrochemical cell involves two half cells one containing an An oxidation-reduction (redox) reaction involves oxidising agent and the other a reducing agent. the transfer of electrons from the reducing agent These cells are connected with a to the oxidising agent. wire, to allow electron flow and a salbdlt bridge to compl ete th e circuit and maintain electrical neutrality. The PULL or DRIVING FORCE on the electrons is the cell OXIDATION - is the LOSS of electrons Zn(s) Zn2+(aq) + 2e-(aq) potential (Ecell) or the REDUCTION - is the GAIN of electrons Cu2+(aq) + 2e-(aq) Cu(s) electromotive force (emf) of the cell, measured in volts. These represents the redox HALF-EQUATIONS 1 2 Electrochemical Cells Balancing Redox Equations An ox, red cat Anode: oxidation The concept of Oxidation Number is artificial. In simple ions it Voltaic (Galvanic) cells are those Reduction: cathode in which spontaneous chemical is equivalent to the charge on the ion. reactions produce electricity and Oxidation involves an increase in oxidation number supply it to other circuits. Reduction involves a decrease in oxidation number G < 0 As half cells, determine oxidation numbers and balance electrons. Electrolytic cells are those in Combine half cells balancing gain/loss of electrons. which electrical energy causes + - Balance with H O and H or H O and OH . non-spontaneous chemical 2 2 reactions to occur. Check charges balance. G > 0 0 +II Zn(s) Zn2+(aq) + 2e-(aq) +II 0 Cu2+(aq) + 2e-(aq) Cu(s) Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) 3 4 Standard Reduction Potentials Calculating Cell Potential In data tables half cells are written as reductions.