Reductions and Reducing Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Process for Purification of Ethylene Compound Having Fluorine-Containing Organic Group
Office europeen des brevets (fi) Publication number : 0 506 374 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 92302586.0 @ Int. CI.5: C07C 17/38, C07C 21/18 (g) Date of filing : 25.03.92 (30) Priority : 26.03.91 JP 86090/91 (72) Inventor : Kishita, Hirofumi 3-19-1, Isobe, Annaka-shi Gunma-ken (JP) (43) Date of publication of application : Inventor : Sato, Shinichi 30.09.92 Bulletin 92/40 3-5-5, Isobe, Annaka-shi Gunma-ken (JP) Inventor : Fujii, Hideki (84) Designated Contracting States : 3-12-37, Isobe, Annaka-shi DE FR GB Gunma-ken (JP) Inventor : Matsuda, Takashi 791 ~4 YdndSG Anri3k3~shi @ Applicant : SHIN-ETSU CHEMICAL CO., LTD. Gunma-ken (JP) 6-1, Ohtemachi 2-chome Chiyoda-ku Tokyo (JP) @) Representative : Votier, Sidney David et al CARPMAELS & RANSFORD 43, Bloomsbury Square London WC1A 2RA (GB) (54) Process for purification of ethylene compound having fluorine-containing organic group. (57) A process for purifying an ethylene compound having a fluorine-containing organic group (fluorine- containing ethylene compound) by mixing the fluorine-containing ethylene compound with an alkali metal or alkaline earth metal reducing agent, and subjecting the resulting mixture to irradiation with ultraviolet radiation, followed by washing with water. The purification process ensures effective removal of iodides which are sources of molecular iodine, from the fluorine-containing ethylene compound. < h- CO CO o 10 o Q_ LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 506 374 A1 BACKGROUND OF THE INVENTION 1. Field of the Invention 5 The present invention relates to a process for purifying an ethylene compound having a fluorine-containing organic group, and more particularly to a purification process by which iodine contained as impurity in an ethylene compound having a fluorine-containing organic group can be removed effectively. -
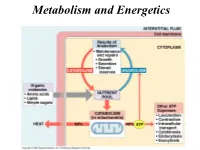
Metabolism and Energetics Oxidation of Carbon Atoms of Glucose Is the Major Source of Energy in Aerobic Metabolism
Metabolism and Energetics Oxidation of carbon atoms of glucose is the major source of energy in aerobic metabolism C6H1206 + 6O2 yields 6 CO2 + H20 + energy Energy released ΔG = - 686 kcal/mol Glucose oxidation requires over 25 discrete steps, with production of 36 ATP. Energy Transformations The mitochondrial synthesis of ATP is not stochiometric. Electron–motive force Proton-motive force Phosphoryl-transfer potential in the form of ATP. Substrate level phosphorylation The formation of ATP by substrate-level phosphorylation ADP ATP P CH O P CH2 O P 2 is used to represent HC OH HC OH a phosphate ester: phosphoglycerate CH OH OH CH2 O P kinase 2 P O bisphosphoglycerate 3-phosphoglycerate OH ADP ATP CH2 CH2 CH3 C O P C OH C O pyruvate kinase non-enzymic COOH COOH COOH phosphoenolpyruvate enolpyruvate pyruvate Why ATP? The reaction of ATP hydrolysis is very favorable ΔGo = - 30.5 kJ/mol = - 7.3 kCal/mol 1. Charge separation of closely packed phosphate groups provides electrostatic relief. Mg2+ 2. Inorganic Pi, the product of the reaction, is immediately resonance-stabilized (electron density spreads equally to all oxygens). 3. ADP immediately ionizes giving H+ into a low [H+] environment (pH~7). 4. Both Pi and ADP are more favorably solvated by water than one ATP molecule. 5. ATP is water soluble. The total body content of ATP and ADP is under 350 mmol – about 10 g, BUT … the amount of ATP synthesized and used each day is about 150 mol – about 110 kg. ATP Production - stage 1 - Glycolysis Glycolysis When rapid production of ATP is needed. -

Chapter 13.Pptx
Chapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic O C C C N C N C X O nitro alkane X= F, Cl, Br, I amines Alkenes Alkyl Halide Chapter 23 OH C C H O C O C C O C C Alkynes phenol alcohols ethers epoxide acidic Chapter 14 H H H C S C C C C S S C C S C C H C C sulfides thiols disulfide H H (thioethers) Arenes 253 Nomenclature of alcohols 1. In general, alcohols are named in the same manner as alkanes; replace the -ane suffix for alkanes with an -ol for alcohols CH3CH2CH2CH3 CH3CH2CH2CH2OH OH butane 1-butanol 2-butanol butan-1-ol butan-2-ol 2. Number the carbon chain so that the hydroxyl group gets the lowest number 3. Number the substituents and write the name listing the substituents in alphabetical order. Many alcohols are named using non-systematic nomenclature H C OH 3 OH OH C OH OH HO OH H3C HO H3C benzyl alcohol allyl alcohol tert-butyl alcohol ethylene glycol glycerol (phenylmethanol) (2-propen-1-ol) (2-methyl-2-propanol) (1,2-ethanediol) (1,2,3-propanetriol) 254 127 Alcohols are classified according to the H R C OH C OH H H degree of substitution of the carbon bearing H H 1° carbon the -OH group methanol primary alcohol primary (1°) : one alkyl substituent R R C OH C OH R R secondary (2°) : two alkyl substituents H R 2° carbon 3° carbon tertiary (3°) : three alkyl substituents secondary alcohol tertiary alcohol Physical properties of alcohols – the C-OH bond of alcohols has a significant dipole moment. -

The First General Electron Transfer Reductions of Carboxylic Acid Derivatives Using Samarium Diiodide
The First General Electron Transfer Reductions of Carboxylic Acid Derivatives Using Samarium Diiodide A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy In the faculty of Engineering and Physical Sciences 2014 Malcolm Peter Spain School of Chemistry Malcolm Spain PhD Thesis Contents Abstract .................................................................................................................... 5 Declaration................................................................................................................ 6 Copyright statement .................................................................................................. 7 Acknowledgements ................................................................................................... 9 Abbreviations .......................................................................................................... 10 Chapter 1. Introduction ........................................................................................... 13 1.1 Introduction to samarium diiodide .................................................................. 13 1.2 Reduction of ketones and aldehydes ............................................................. 15 1.3 Reduction of carboxylic acid derivatives ....................................................... 23 Chapter 2. Investigations of the SmI2–H2O system ................................................. 27 2.0 Preliminary studies of the SmI2–H2O system ................................................ -

Radical Approaches to Alangium and Mitragyna Alkaloids
Radical Approaches to Alangium and Mitragyna Alkaloids A Thesis Submitted for a PhD University of York Department of Chemistry 2010 Matthew James Palframan Abstract The work presented in this thesis has focused on the development of novel and concise syntheses of Alangium and Mitragyna alkaloids, and especial approaches towards (±)-protoemetinol (a), which is a key precursor of a range of Alangium alkaloids such as psychotrine (b) and deoxytubulosine (c). The approaches include the use of a key radical cyclisation to form the tri-cyclic core. O O O N N N O O O H H H H H H O N NH N Protoemetinol OH HO a Psychotrine Deoxytubulosine b c Chapter 1 gives a general overview of radical chemistry and it focuses on the application of radical intermolecular and intramolecular reactions in synthesis. Consideration is given to the mediator of radical reactions from the classic organotin reagents, to more recently developed alternative hydrides. An overview of previous synthetic approaches to a range of Alangium and Mitragyna alkaloids is then explored. Chapter 2 follows on from previous work within our group, involving the use of phosphorus hydride radical addition reactions, to alkenes or dienes, followed by a subsequent Horner-Wadsworth-Emmons reaction. It was expected that the tri-cyclic core of the Alangium alkaloids could be prepared by cyclisation of a 1,7-diene, using a phosphorus hydride to afford the phosphonate or phosphonothioate, however this approach was unsuccessful and it highlighted some limitations of the methodology. Chapter 3 explores the radical and ionic chemistry of a range of silanes. -

United States Patent 0
1 3,667,923 United States Patent 0 ice Patented June 6, 1972 1 2 ‘I have discovered that anhydrous hydrogen cyanide $667,923 reacts readily with a quaternary ammonium borohydride PREPARATION OF LITHIUM, SODIUM AND and with the borohydrides of lithium and sodium at QUATERNARY AMMONIUM CYANOBORO atmospheric pressure in solvents for the borohydride, such HYDRIDES Robert C. Wade, Ipswich, Mass, assignor to Ventron as glyme, diglyme, triglyme, tetrahydrofuran and dimethyl Corporation, Beverly, Mass. formamide, or mixtures of these at a temperatures be No Drawing. Filed June 16, 1969, Ser. No. 833,722 tween 0° and the boiling point of the solvent to form Int. Cl. C01c 3/08; C01!) 35/00 the corresponding cyanoborohydride. The preferred sol US. Cl. 23-358 4 Claims vents are tetrahydrofuran and glyme because of their 10 convenient boiling points. Potassium borohydride does not react readily with ABSTRACT OF THE DISCLOSURE hydrogen cyanide in tetrahydrofuran or glyme presum The invention relates to the preparation of lithium, ably because of its lack of solubility in these solvents. sodium and quaternary ammonium cyanoborohydrides. However, it reacts with hydrogen cyanide in dimethyl These compounds are prepared by mixing substantially formamide similarly to the other borohydrides mentioned anhydrous hydrogen cyanide with a substantially an above. hydrous lithium or sodium or quaternary ammonium The reaction of the process of the invention appears to borohydride at a temperature between 0° C. and 100° C. take place in two stages. Thus, if the reaction mixture is in a substantially anhydrous solvent, such as tetrahydro initially maintained between about 10° and about 35° C. -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

SAFETY DATA SHEET Revision Date 15.09.2021 According to Regulation (EC) No
Version 6.6 SAFETY DATA SHEET Revision Date 15.09.2021 according to Regulation (EC) No. 1907/2006 Print Date 24.09.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Lithium borohydride Product Number : 222356 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 16949-15-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Substances and mixtures which in contact with water emit flammable gases (Category 1), H260 Acute toxicity, Oral (Category 3), H301 Skin corrosion (Sub-category 1B), H314 For the full text of the H-Statements mentioned in this Section, see Section 16. Aldrich- 222356 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal word Danger Hazard statement(s) H260 In contact with water releases flammable gases which may ignite spontaneously. -

UNIT 6 ELEMENTS of GROUP 13 Structure 6.1 Introduction Objectives I 6.2 Oailcrence, Extraction and Uses Occurrec - Extraction Uses I 6.3 General Characteristics
UNIT 6 ELEMENTS OF GROUP 13 structure 6.1 Introduction Objectives I 6.2 Oailcrence, Extraction and Uses Occurrec - Extraction uses i 6.3 General Characteristics I - 6.5 Halides of Bpron anel Aluminium Halides of Boron Halides of Aluminium 6.6 Oxides of Boron and Aluminium I Boric Oxide Aluminium Oxide 6.7 Oxoacids of Boron and Borates 6.8 Borazine 6.9 Complexation Behaviour 6.10 Anomalous Behaviour of Boron 6.11 Summary 6.12 Terminal Questions 6.13- Answers ' -- 6.1 INTRODUCTION In the previous two units, you studied the main features of the chemistry of Group 1 and Group 2 elements, i.e. the alkali and the alkaline earth metals. In this unit you - will study the elements of Group 13, namely, boron, aluminium, gallium, indium and, thallium. While studying the alkali and alkaline earth metals, you have seen that all Zhe elements of these two groups are highly reactive metals and the first element of each group shows some differences from the rest. In Group 13, the differences between the first element and the remaining elements become so pronounced that the first member of the group, i.e. boron is a nonmetal wheieas the rest of the elements are distinctly metallic in nature. In a way, this is the first group of the periodic table in which you observe a marked change in the hature of the elements . down the group. describe the chemistry of hydrides, halides and oxides of boron and aluminium, elucidate the structures of hydrides of boron and aluminium, 6.2 OCCURRENC~,EXTRACTION AND USES Elements bf Group 13 are sufficiently reactive. -

Chapter 20 Electrochemistry
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method of half-reactions. Sketch a voltaic cell and identify its cathode, anode, and the directions in which electrons and ions move. o Calculate standard emfs (cell potentials), E cell, from standard reduction potentials. Use reduction potentials to predict whether a redox reaction is spontaneous. o o Relate E cell to DG and equilibrium constants. Calculate emf under nonstandard conditions. Identify the components of common batteries. Describe the construction of a lithium-ion battery and explain how it works. Describe the construction of a fuel cell and explain how it generates electrical energy. Explain how corrosion occurs and how it is prevented by cathodic protection. Describe the reactions in electrolytic cells. Relate the amounts of products and reactants in redox reactions to electrical charge. Electrochemistry Electrochemistry is the study of the relationships between electricity and chemical reactions. • It includes the study of both spontaneous and nonspontaneous processes. 1 Redox reactions: assigning oxidation numbers Oxidation numbers help keep track of what species loses electrons and what species gains them. • An element is oxidized when the oxidation number increases • An element is reduced when the oxidation number decreases • an oxidizing agent causes another element to be oxidized • a reducing agent causes another element to be reduced. Assigning oxidation numbers (sect. 4.4) 1. Elemental form, each atom has ox. # = 0. Zn O2 O3 I2 S8 P4 2. Simple ions, = charge on ion. -1 for Cl-, +2 for Mg2+ 3. -

Ch. 21.1 Redox Reactions and Electrochemical Cells
Pre-Health Post-Baccalaureate Program Study Guide and Practice Problems Course: CHM2046 Textbook Chapter: 21.1 (Silberberg 6e) Topics Covered: Redox Reactions and Electrochemical Cells Created by Isaac Loy 1. Review Understanding this chapter’s material will depend on an in- depth understanding of redox reactions, which were first covered last semester in ch. 4. We will review redox reactions in this study guide, but it would be wise to review ch. 4 if you are having difficulty with this material. Redox reactions will also be incredibly important moving forward into organic chemistry and biochemistry. 2. Oxidation-Reduction Reactions The mnemonic that you will come back to time and time again for this topic is: “LEO the lion says GER” Where “LEO” stands for Loss of Electrons = Oxidation And “GER” stands for Gain of Electrons = Reduction The oxidizing agent (the substance that is being reduced) pulls electrons from the substance that is being oxidized. The reducing agent (the substance that is being oxidized) gives electrons to the substance that is being reduced. Oxidation and reduction are simultaneous processes. In order for a redox reaction to take place, one substance must be oxidized and the other must be reduced. When working with oxidation numbers to solve problems, the substance being oxidized (LEO -> loss of electrons) becomes more positive. Likewise, the substance being reduced (GER -> gaining electrons) becomes more negative. 3. Using Half-Reactions to Solve Redox Problems Follow the steps below to create half-reactions. It is crucial that you follow the steps in order! A. Split up the overall reaction into two half-reactions, where the species of one reaction is being oxidized, and the species of the other reaction is being reduced. -

CHE202 Reductions and Heterocycles
CHE202 Structure & Reactivity in Organic Chemistry: ! Reduction Reactions and Heterocyclic Chemistry! 9 lectures, Semester B 2014! Dr. Chris Jones! ! [email protected]! Office: 1.07 Joseph Priestley Building! ! Office hours:! 9.30-10.30 am Tuesday! 1.30-2.30 pm Thursday (by appointment only)! Course structure and recommended texts! 2! §" Coursework:! !Semester B – week 9 ! !5% (‘Coursework 7’)! !Semester B – week 11! !5% (‘Coursework 8’)! ! §" Test:! !Semester B – week 12! !15% (‘Test 4’)! §" Recommended text books:! ‘Organic Chemistry’, Clayden, ‘Oxidation & Reduction in ‘Heterocyclic Chemistry’, Greeves & Warren, OUP, 2012.! Organic Chemistry’, Donohoe, Joule & Mills, Wiley, 2010.! OUP, 2000.! Don’t forget clickers! Overview of Reduction Chemistry lecture material! 3! §" Reduction:! - Definition (recap.)! - Reduction of carbon-carbon double and triple bonds! - Heterogeneous hydrogenation! - Homogeneous hydrogenation, including stereoselective hydrogenation! - Dissolved metal reductions! - Other methods of reduction! - Reduction of carbon-heteroatom double and triple bonds! - Reduction of carbonyl derivatives, addressing chemoselectivity! - Stereoselective reduction of carbonyl derivatives! - Reduction of imines and nitriles! - Reductive cleavage reactions! - Hydrogenolysis of benzyl and allyl groups! - Dissolved metal reduction! - Deoxygenation reactions! - Reduction of heteroatom functional groups! e.g. azides, nitro groups, N-O bond cleavage! Reduction: definition! 4! §" Reduction of an organic substrate can be defined as:! - The concerted