Redox Reactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redox Models in Chemistry
Redox models in chemistry A depiction of the conceptions held by secondary school students of redox reactions Lise-Lotte Österlund Department of Chemistry 901 87 Umeå Umeå 2010 Copyright©Lise-Lotte Österlund ISBN: 978-91-7459-053-1 ISSN: 1652-5051 Cover picture: Photographer, Lise-Lotte Österlund Printed by: VMC, KBC, Umeå University Umeå, Sweden 2010 T0 my family Abstract According to previous research, students show difficulties in learning redox reactions. By the historical development different redox models exist to explain redox reactions, the oxygen model, the hydrogen model, the electron model and the oxidation number model. This thesis reports about three studies concerning conceptions held by secondary school students of redox reactions. A textbook analysis is also included in the thesis. The first study was an investigation of the students’ use of redox models in inorganic contexts, their use of the activity series of metals, and the students’ ability to transfer redox knowledge. Then the students’ work with an open- ended biochemical task, where the students had access of the textbook was studied. The students talk about redox reactions, the questions raised by the students, what resources used to answer the questions and what kind of talk developed were investigated. A textbook analysis based on chemistry books from Sweden and one book from England was performed. The redox models used as well as the dealing with redox related learning difficulties was studied. Finally, the students’ conceptions about redox in inorganic, organic and biochemistry after completed chemistry courses were studied. The results show that the students were able to use the electron model as a tool to explain inorganic redox reactions and the mutuality of oxidation and reduction was fundamental. -

Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth
Subscriber access provided by RES CENTER OF ECO ENVIR SCI Article Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth Metals on Cobalt#Cerium Composite Oxide Catalysts for N2O Decomposition Li Xue, Hong He, Chang Liu, Changbin Zhang, and Bo Zhang Environ. Sci. Technol., 2009, 43 (3), 890-895 • DOI: 10.1021/es801867y • Publication Date (Web): 05 January 2009 Downloaded from http://pubs.acs.org on January 31, 2009 More About This Article Additional resources and features associated with this article are available within the HTML version: • Supporting Information • Access to high resolution figures • Links to articles and content related to this article • Copyright permission to reproduce figures and/or text from this article Environmental Science & Technology is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036 Environ. Sci. Technol. 2009, 43, 890–895 Promotion Effects and Mechanism such as Fe-ZSM-5 are more active in the selective catalytic reduction (SCR) of N2O by hydrocarbons than in the - ° of Alkali Metals and Alkaline Earth decomposition of N2O in a temperature range of 300 400 C (3). In recent years, it has been found that various mixed Metals on Cobalt-Cerium oxide catalysts, such as calcined hydrotalcite and spinel oxide, showed relatively high activities. Composite Oxide Catalysts for N2O One of the most active oxide catalysts is a mixed oxide containing cobalt spinel. Calcined hydrotalcites containing Decomposition cobalt, such as Co-Al-HT (9-12) and Co-Rh-Al-HT (9, 11), have been reported to be very efficient for the decomposition 2+ LI XUE, HONG HE,* CHANG LIU, of N2O. -

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -

Process for Purification of Ethylene Compound Having Fluorine-Containing Organic Group
Office europeen des brevets (fi) Publication number : 0 506 374 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 92302586.0 @ Int. CI.5: C07C 17/38, C07C 21/18 (g) Date of filing : 25.03.92 (30) Priority : 26.03.91 JP 86090/91 (72) Inventor : Kishita, Hirofumi 3-19-1, Isobe, Annaka-shi Gunma-ken (JP) (43) Date of publication of application : Inventor : Sato, Shinichi 30.09.92 Bulletin 92/40 3-5-5, Isobe, Annaka-shi Gunma-ken (JP) Inventor : Fujii, Hideki (84) Designated Contracting States : 3-12-37, Isobe, Annaka-shi DE FR GB Gunma-ken (JP) Inventor : Matsuda, Takashi 791 ~4 YdndSG Anri3k3~shi @ Applicant : SHIN-ETSU CHEMICAL CO., LTD. Gunma-ken (JP) 6-1, Ohtemachi 2-chome Chiyoda-ku Tokyo (JP) @) Representative : Votier, Sidney David et al CARPMAELS & RANSFORD 43, Bloomsbury Square London WC1A 2RA (GB) (54) Process for purification of ethylene compound having fluorine-containing organic group. (57) A process for purifying an ethylene compound having a fluorine-containing organic group (fluorine- containing ethylene compound) by mixing the fluorine-containing ethylene compound with an alkali metal or alkaline earth metal reducing agent, and subjecting the resulting mixture to irradiation with ultraviolet radiation, followed by washing with water. The purification process ensures effective removal of iodides which are sources of molecular iodine, from the fluorine-containing ethylene compound. < h- CO CO o 10 o Q_ LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 506 374 A1 BACKGROUND OF THE INVENTION 1. Field of the Invention 5 The present invention relates to a process for purifying an ethylene compound having a fluorine-containing organic group, and more particularly to a purification process by which iodine contained as impurity in an ethylene compound having a fluorine-containing organic group can be removed effectively. -
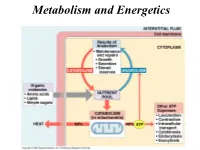
Metabolism and Energetics Oxidation of Carbon Atoms of Glucose Is the Major Source of Energy in Aerobic Metabolism
Metabolism and Energetics Oxidation of carbon atoms of glucose is the major source of energy in aerobic metabolism C6H1206 + 6O2 yields 6 CO2 + H20 + energy Energy released ΔG = - 686 kcal/mol Glucose oxidation requires over 25 discrete steps, with production of 36 ATP. Energy Transformations The mitochondrial synthesis of ATP is not stochiometric. Electron–motive force Proton-motive force Phosphoryl-transfer potential in the form of ATP. Substrate level phosphorylation The formation of ATP by substrate-level phosphorylation ADP ATP P CH O P CH2 O P 2 is used to represent HC OH HC OH a phosphate ester: phosphoglycerate CH OH OH CH2 O P kinase 2 P O bisphosphoglycerate 3-phosphoglycerate OH ADP ATP CH2 CH2 CH3 C O P C OH C O pyruvate kinase non-enzymic COOH COOH COOH phosphoenolpyruvate enolpyruvate pyruvate Why ATP? The reaction of ATP hydrolysis is very favorable ΔGo = - 30.5 kJ/mol = - 7.3 kCal/mol 1. Charge separation of closely packed phosphate groups provides electrostatic relief. Mg2+ 2. Inorganic Pi, the product of the reaction, is immediately resonance-stabilized (electron density spreads equally to all oxygens). 3. ADP immediately ionizes giving H+ into a low [H+] environment (pH~7). 4. Both Pi and ADP are more favorably solvated by water than one ATP molecule. 5. ATP is water soluble. The total body content of ATP and ADP is under 350 mmol – about 10 g, BUT … the amount of ATP synthesized and used each day is about 150 mol – about 110 kg. ATP Production - stage 1 - Glycolysis Glycolysis When rapid production of ATP is needed. -

Reductions and Reducing Agents
REDUCTIONS AND REDUCING AGENTS 1 Reductions and Reducing Agents • Basic definition of reduction: Addition of hydrogen or removal of oxygen • Addition of electrons 9:45 AM 2 Reducible Functional Groups 9:45 AM 3 Categories of Common Reducing Agents 9:45 AM 4 Relative Reactivity of Nucleophiles at the Reducible Functional Groups In the absence of any secondary interactions, the carbonyl compounds exhibit the following order of reactivity at the carbonyl This order may however be reversed in the presence of unique secondary interactions inherent in the molecule; interactions that may 9:45 AM be activated by some property of the reacting partner 5 Common Reducing Agents (Borohydrides) Reduction of Amides to Amines 9:45 AM 6 Common Reducing Agents (Borohydrides) Reduction of Carboxylic Acids to Primary Alcohols O 3 R CO2H + BH3 R O B + 3 H 3 2 Acyloxyborane 9:45 AM 7 Common Reducing Agents (Sodium Borohydride) The reductions with NaBH4 are commonly carried out in EtOH (Serving as a protic solvent) Note that nucleophilic attack occurs from the least hindered face of the 8 carbonyl Common Reducing Agents (Lithium Borohydride) The reductions with LiBH4 are commonly carried out in THF or ether Note that nucleophilic attack occurs from the least hindered face of the 9:45 AM 9 carbonyl. Common Reducing Agents (Borohydrides) The Influence of Metal Cations on Reactivity As a result of the differences in reactivity between sodium borohydride and lithium borohydride, chemoselectivity of reduction can be achieved by a judicious choice of reducing agent. 9:45 AM 10 Common Reducing Agents (Sodium Cyanoborohydride) 9:45 AM 11 Common Reducing Agents (Reductive Amination with Sodium Cyanoborohydride) 9:45 AM 12 Lithium Aluminium Hydride Lithium aluminiumhydride reacts the same way as lithium borohydride. -

Chapter 20 Electrochemistry
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method of half-reactions. Sketch a voltaic cell and identify its cathode, anode, and the directions in which electrons and ions move. o Calculate standard emfs (cell potentials), E cell, from standard reduction potentials. Use reduction potentials to predict whether a redox reaction is spontaneous. o o Relate E cell to DG and equilibrium constants. Calculate emf under nonstandard conditions. Identify the components of common batteries. Describe the construction of a lithium-ion battery and explain how it works. Describe the construction of a fuel cell and explain how it generates electrical energy. Explain how corrosion occurs and how it is prevented by cathodic protection. Describe the reactions in electrolytic cells. Relate the amounts of products and reactants in redox reactions to electrical charge. Electrochemistry Electrochemistry is the study of the relationships between electricity and chemical reactions. • It includes the study of both spontaneous and nonspontaneous processes. 1 Redox reactions: assigning oxidation numbers Oxidation numbers help keep track of what species loses electrons and what species gains them. • An element is oxidized when the oxidation number increases • An element is reduced when the oxidation number decreases • an oxidizing agent causes another element to be oxidized • a reducing agent causes another element to be reduced. Assigning oxidation numbers (sect. 4.4) 1. Elemental form, each atom has ox. # = 0. Zn O2 O3 I2 S8 P4 2. Simple ions, = charge on ion. -1 for Cl-, +2 for Mg2+ 3. -

Redox Potential Measurements M.J
Redox Potential Measurements M.J. Vepraskas NC State University December 2002 Redox potential is an electrical measurement that shows the tendency of a soil solution to transfer electrons to or from a reference electrode. From this measurement we can estimate whether the soil is aerobic, anaerobic, and whether chemical compounds such as Fe oxides or nitrate have been chemically reduced or are present in their oxidized forms. Making these measurements requires three basic pieces of equipment: 1. Platinum electrode 2. Voltmeter 3. Reference electrode The basic set-up is shown below: Voltmeter 456 mv Soil surface Reference Pt wire electrode Fig. 1. The Pt wire is buried into the soil to be in contact with the soil solution. The reference electrode must also be in contact with the soil solution. It has a ceramic tip, which can be placed in the soil, or the electrode can be placed in a salt bridge, which is itself placed in the soil. Wires from both the Pt electrode and reference electrode are connected to the voltmeter. Pt Electrodes Platinum electrodes consist of a small piece of platinum wire that is soldered or fused to wire made another metal. Platinum conducts electrons from the soil solution to the wire to which it is attached. Platinum is used because it is assumed to be an inert metal. This means it does not give up its own electrons (does not oxidize) to the wire or soil solution. Iron containing materials such as steel will oxidize themselves and send their own electrons to the voltmeter. As a result the voltage we measure will not result solely from electrons being transferred to or from the soil. -

Ch. 21.1 Redox Reactions and Electrochemical Cells
Pre-Health Post-Baccalaureate Program Study Guide and Practice Problems Course: CHM2046 Textbook Chapter: 21.1 (Silberberg 6e) Topics Covered: Redox Reactions and Electrochemical Cells Created by Isaac Loy 1. Review Understanding this chapter’s material will depend on an in- depth understanding of redox reactions, which were first covered last semester in ch. 4. We will review redox reactions in this study guide, but it would be wise to review ch. 4 if you are having difficulty with this material. Redox reactions will also be incredibly important moving forward into organic chemistry and biochemistry. 2. Oxidation-Reduction Reactions The mnemonic that you will come back to time and time again for this topic is: “LEO the lion says GER” Where “LEO” stands for Loss of Electrons = Oxidation And “GER” stands for Gain of Electrons = Reduction The oxidizing agent (the substance that is being reduced) pulls electrons from the substance that is being oxidized. The reducing agent (the substance that is being oxidized) gives electrons to the substance that is being reduced. Oxidation and reduction are simultaneous processes. In order for a redox reaction to take place, one substance must be oxidized and the other must be reduced. When working with oxidation numbers to solve problems, the substance being oxidized (LEO -> loss of electrons) becomes more positive. Likewise, the substance being reduced (GER -> gaining electrons) becomes more negative. 3. Using Half-Reactions to Solve Redox Problems Follow the steps below to create half-reactions. It is crucial that you follow the steps in order! A. Split up the overall reaction into two half-reactions, where the species of one reaction is being oxidized, and the species of the other reaction is being reduced. -

Gen Chem II Jasperse Ch. 19 Electrochemistry 1
Gen Chem II Jasperse Ch. 19 Electrochemistry 1 Chapter 19 Electrochemistry Math Summary Relating Standard Cell Potential to Standard Half Cell Potentials Eºcell=Eºoxidation + Eºreduction (standard conditions assume 1.0 M concentrations) Relating Half Cell Potentials when Written in Opposite Directions Eºox = -Eºred for half reactions written in opposite directions Relating Standard Cell Potentials to ∆G ∆Gº = -nFE˚cell (to give answer in kJ, use F = 96.485) F = 96,500 C/mol n=number of electrons transferred Relating Actual Cell Potential to Standard Cell Potential when Concentrations aren't 1.0-M Ecell = Eºcell -[0.0592/n] log Q (Q = ratio of actual concentrations) Relating Standard Cell Potential to Equilibrium Constant log K = nEº/0.0592 Relating Actual Cell Potential to Actual Concentrations in Concentration Cells Ecell = -[0.0592/n] log Q for concentration cells, where anode and cathode differ only in concentration, but otherwise have same ions Relating # of Moles of Electrons Transferred as a Function of Time and Current in Electrolysis 1 mol e- = 96,500 C moles of electrons = [current (A)•time (sec)]/96,500 for electrolysis, moles, current, and time are related. rearranged: time (sec)=(moles of electrons)(96500)/current (in A) Note: 3600 sec/hour so time (hours)=(moles of electrons)(26.8)/current (in A) Electrochemistry-Related Units C = Coulomb = quantity of electrical charge = 6.24 • 1018 electrons • 1 mole of electrons = 96,500 C A = amp = rate of charge flow per time = C/sec V = volt = electrical power/force/strength = J/C 96,500C 96.5 kJ F = Faraday = = mole e− mole e− •V € € Gen Chem II Jasperse Ch. -

Origins of Life in the Universe Zackary Johnson
11/4/2007 Origins of Life in the Universe Zackary Johnson OCN201 Fall 2007 [email protected] Zackary Johnson http://www.soest.hawaii.edu/oceanography/zij/education.html Uniiiversity of Hawaii Department of Oceanography Class Schedule Nov‐2Originsof Life and the Universe Nov‐5 Classification of Life Nov‐7 Primary Production Nov‐9Consumers Nov‐14 Evolution: Processes (Steward) Nov‐16 Evolution: Adaptation() (Steward) Nov‐19 Marine Microbiology Nov‐21 Benthic Communities Nov‐26 Whale Falls (Smith) Nov‐28 The Marine Food Web Nov‐30 Community Ecology Dec‐3 Fisheries Dec‐5Global Ecology Dec‐12 Final Major Concepts TIMETABLE Big Bang! • Life started early, but not at the beginning, of Earth’s Milky Way (and other galaxies formed) history • Abiogenesis is the leading hypothesis to explain the beginning of life on Earth • There are many competing theories as to how this happened • Some of the details have been worked out, but most Formation of Earth have not • Abiogenesis almost certainly occurred in the ocean 20‐15 15‐94.5Today Billions of Years Before Present 1 11/4/2007 Building Blocks TIMETABLE Big Bang! • Universe is mostly hydrogen (H) and helium (He); for Milky Way (and other galaxies formed) example –the sun is 70% H, 28% He and 2% all else! Abundance) e • Most elements of interest to biology (C, N, P, O, etc.) were (Relativ 10 produced via nuclear fusion Formation of Earth Log at very high temperature reactions in large stars after Big Bang 20‐13 13‐94.7Today Atomic Number Billions of Years Before Present ORIGIN OF LIFE ON EARTH Abiogenesis: 3 stages Divine Creation 1. -

Evolution of the First Metabolic Cycles
Proc. Natl. Acad. Sci. USA Vol. 87, pp. 200-204, January 1990 Evolution Evolution of the first metabolic cycles (chemoautotrophy/reductive citric acid cycle/origin of life/pyrite) GUNTER WACHTERSHAUSER 8000 Munich 2, Tal 29, Federal Republic of Germany Communicated by Karl Popper, October 12, 1989 (received for review February 28, 1989) ABSTRACT There are two alternatives concerning the genobacter thermophilus (13), and Desulfobacter hydro- origin of life: the origin may be heterotrophic or autotrophic. genophilus (14) and also in the sulfur-associated archaebac- The central problem within the theory of an autotrophic origin teria Thermoproteus neutrophilus (15) and, partly demon- is the first process of carbon fixation. I here propose the strated, in Sulfolobus brierleyi (16). As suggested by Kandler hypothesis that this process is an autocatalytic cycle that can be and Stetter (16) and previously by Hartmann (17), it may be retrodictively constructed from the extant reductive citric acid considered to be of great antiquity and the evolutionary cycle by replacing thioesters by thioacids and by assuming that precursor ofthe oxidative Krebs cycle. It is here conjectured the required reducing power is obtained from the oxidative to be the extant candidate for the reconstruction of the formation of pyrite (FeS2). This archaic cycle is strictly archaic autocatalytic cycle of carbon fixation. chemoautotrophic: photoautotrophy is not required. The cycle The presently accepted form of the extant reductive citric is catalytic for pyrite formation and autocatalytic for its own acid cycle is shown in Fig. 1 in a somewhat unusual repre- multiplication. It is a consequence of this hypothesis that the sentation, twisted in an 8.