Reproductive Physiology of Bonefishes (Albula Spp.) Across the Northwest Bahamas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

V a Tion & Management of Reef Fish Sp a Wning Aggrega Tions
handbook CONSERVATION & MANAGEMENT OF REEF FISH SPAWNING AGGREGATIONS A Handbook for the Conservation & Management of Reef Fish Spawning Aggregations © Seapics.com Without the Land and the Sea, and their Bounties, the People and their Traditional Ways would be Poor and without Cultural Identity Fijian Proverb Why a Handbook? 1 What are Spawning Aggregations? 2 How to Identify Spawning Aggregations 2 Species that Aggregate to Spawn 2 Contents Places Where Aggregations Form 9 Concern for Spawning Aggregations 10 Importance for Fish and Fishermen 10 Trends in Exploited Aggregations 12 Managing & Conserving Spawning Aggregations 13 Research and Monitoring 13 Management Options 15 What is SCRFA? 16 How can SCRFA Help? 16 SCRFA Work to Date 17 Useful References 18 SCRFA Board of Directors 20 Since 2000, scientists, fishery managers, conservationists and politicians have become increasingly aware, not only that many commercially important coral reef fish species aggregate to spawn (reproduce) but also that these important reproductive gatherings are particularly susceptible to fishing. In extreme cases, when fishing pressure is high, aggregations can dwindle and even cease to form, sometimes within just a few years. Whether or not they will recover and what the long-term effects on the fish population(s) might be of such declines are not yet known. We do know, however, that healthy aggregations tend to be associated with healthy fisheries. It is, therefore, important to understand and better protect this critical part of the life cycle of aggregating species to ensure that they continue to yield food and support livelihoods. Why a Handbook? As fishing technology improved in the second half of the twentieth century, engines came to replace sails and oars, the cash economy developed rapidly, and human populations and demand for seafood grew, the pressures on reef fishes for food, and especially for money, increased enormously. -

The Need for Sustainable Management of Coral Reef Fish Spawning Aggregations
Secretariat of the Pacific Community Seventh Heads of Fisheries Meeting (28 Feb.–4 March 2011, Noumea, New Caledonia) Working Paper 7 Original: English The need for sustainable management of coral reef fish spawning aggregations Yvonne Sadovy PhD The University of Hong-Kong and Eric Clua Coral Reef Initiative for the South Pacific (CRISP), SPC www.spc.int/fame/ SPC/HOF7/Working Paper 7 Page 1 The need for sustainable management of coral reef fish spawning aggregations 1. Many commercially important species of reef fishes exhibit the habit of ‘aggregation-spawning’ whereby all mating takes place in large temporary gatherings of conspecifics. Sometimes fish also migrate in large numbers to spawning sites. These mating-related gatherings and movements are often highly predictable in time and location and hence, once discovered, are often the basis of important seasonal fisheries. Yet, as we have come to discover over the last two decades, such reproductive gatherings are highly vulnerable to over-fishing and, if severely compromised by fishing, may cease to form completely. If this happens adults will no longer produce the eggs and larvae that form the basis of fisheries at non-reproductive times of the year, and the fishery is likely to collapse. Collapse would represent the loss of significant fisheries with important and sometimes serious implications for the human communities that depend on such fishes. Moreover, aggregations can represent significant biomass and movements of animals, and are formed by many species, highlighting the ecosystem importance of these biological events. 2. Species that predictably aggregate to spawn, or gather in large spawning migrations, include a diverse range of fishes of much importance as food and commercial benefit in the Pacific. -

Reproductive Development of Female Bonefish (Albula Spp.)
REPRODUCTIVE DEVELOPMENT OF FEMALE BONEFISH (ALBULA SPP.) FROM THE BAHAMAS by Cameron Alexander Luck A Thesis Submitted to the Faculty of The Charles E. Schmidt College of Science In Partial Fulfilment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2018 Copyright 2018 by Cameron Luck ii ACKNOWLEDGEMENTS I sincerely appreciate the guidance of my advisor Dr. Matthew Ajemian for his continued support throughout this endeavor as well as the input from my committee members: Dr. Sahar Mejri, Dr. Aaron Adams, and Dr. Paul Wills. I am extremely grateful for the collaboration and support of Justin Lewis. Field collections would have been impossible without his local knowledge, willingness to be available, passion for bonefish, and incredible work ethic. I am also grateful for the words of wisdom and comedic relief often provided by fellow graduate students, lab members, and interns of the Fish Ecology and Conservation Lab: Grace Roskar, Breanna Degroot, Steve Lombardo, Rachel Shaw, Mike McCallister, Alex Allen, and many others. This project was financially supported by Bonefish & Tarpon Trust (BTT) and National Fish and Wildlife Foundation (NFWF). We are grateful for the lodging and water access provided by East End Lodge, Andros South, and Hank’s Place. The experimental protocol for this study received approval from Florida Atlantic University’s Institutional Animal Care and Use Committee (Animal Use Protocol #A16-34). iv ABSTRACT Author: Cameron Luck Title: Reproductive Development of Female Bonefish (Albula spp.) from the Bahamas Institution: Florida Atlantic University Thesis Advisor: Dr. Matthew J. Ajemian Degree: Master of Science Year: 2018 Bonefish (Albula spp.) support an economically important sport fishery, yet little is known regarding the reproductive biology of this genus. -

Rhodopsin Gene Evolution in Early Teleost Fishes
RESEARCH ARTICLE Rhodopsin gene evolution in early teleost fishes 1 2 1 Jhen-Nien Chen , Sarah Samadi , Wei-Jen ChenID * 1 Institute of Oceanography, National Taiwan University, Taipei, Taiwan, 2 Institute de SysteÂmatique, E volution, Biodiversite (ISYEB), MuseÂum National d'Histoire Naturelle±CNRS, Sorbonne UniversiteÂ, EPHE, Paris, France * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Rhodopsin mediates an essential step in image capture and is tightly associated with visual adaptations of aquatic organisms, especially species that live in dim light environments (e.g., the deep sea). The rh1 gene encoding rhodopsin was formerly considered a single- copy gene in genomes of vertebrates, but increasing exceptional cases have been found in teleost fish species. The main objective of this study was to determine to what extent the OPEN ACCESS visual adaptation of teleosts might have been shaped by the duplication and loss of rh1 Citation: Chen J-N, Samadi S, Chen W-J (2018) genes. For that purpose, homologous rh1/rh1-like sequences in genomes of ray-finned Rhodopsin gene evolution in early teleost fishes. PLoS ONE 13(11): e0206918. https://doi.org/ fishes from a wide taxonomic range were explored using a PCR-based method, data mining 10.1371/journal.pone.0206918 of public genetic/genomic databases, and subsequent phylogenomic analyses of the Editor: Michael Schubert, Laboratoire de Biologie retrieved sequences. We show that a second copy of the fish-specific intron-less rh1 is pres- du DeÂveloppement de Villefranche-sur-Mer, ent in the genomes of most anguillids (Elopomorpha), Hiodon alosoides (Osteoglossomor- FRANCE pha), and several clupeocephalan lineages. -

Teleost Radiation Teleost Monophyly
Teleost Radiation Teleostean radiation - BIG ~ 20,000 species. Others put higher 30,000 (Stark 1987 Comparative Anatomy of Vertebrates) About 1/2 living vertebrates = teleosts Tetrapods dominant land vertebrates, teleosts dominate water. First = Triassic 240 my; Originally thought non-monophyletic = many independent lineages derived from "pholidophorid" ancestry. More-or-less established teleostean radiation is true monophyletic group Teleost Monophyly • Lauder and Liem support this notion: • 1. Mobile premaxilla - not mobile like maxilla halecostomes = hinged premaxilla modification enhancing suction generation. Provides basic structural development of truly mobile premaxilla, enabling jaw protrusion. Jaw protrusion evolved independently 3 times in teleostean radiation 1) Ostariophysi – Cypriniformes; 2) Atherinomorpha and 2) Percomorpha – especially certain derived percomorphs - cichlids and labroid allies PREMAXILLA 1 Teleost Monophyly • Lauder & Liem support notion: • 2. Unpaired basibranchial tooth plates (trend - consolidation dermal tooth patches in pharynx). • Primitive = whole bucco- pharynx w/ irregular tooth patches – consolidate into functional units - modified w/in teleostei esp. functional pharyngeal jaws. Teleost Monophyly • Lauder and Liem support this notion: • 3. Internal carotid foramen enclosed in parasphenoid (are all characters functional, maybe don't have one - why should they?) 2 Teleost Tails • Most interesting structure in teleosts is caudal fin. • Teleosts possess caudal skeleton differs from other neopterygian fishes - Possible major functional significance in Actinopterygian locomotor patterns. • Halecomorphs-ginglymodes = caudal fin rays articulate with posterior edge of haemal spines and hypurals (modified haemal spines). Fin is heterocercal (inside and out). Ginglymod - Gars Halecomorph - Amia 3 Tails • “Chondrostean hinge” at base of upper lobe - weakness btw body and tail lobe. Asymmetrical tail = asymmetrical thrust with respect to body axis. -
Field Identification Guide to the Living Marine Resources in Kenya
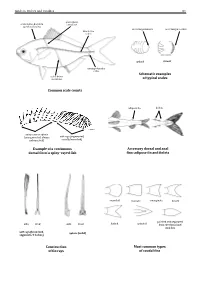
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Hydrodynamic and Isotopic Niche Differentiation Between Juveniles of Two Sympatric Cryptic Bonefishes, Albula Vulpes and Albula Goreensis
Environ Biol Fish (2019) 102:129–145 https://doi.org/10.1007/s10641-018-0810-7 Hydrodynamic and isotopic niche differentiation between juveniles of two sympatric cryptic bonefishes, Albula vulpes and Albula goreensis Christopher R. Haak & Michael Power & Geoffrey W. Cowles & Andy J. Danylchuk Received: 16 February 2018 /Accepted: 20 August 2018 /Published online: 4 October 2018 # Springer Nature B.V. 2018 Abstract We employed numerical wave models, GIS, utilization. Otolith δ18O did not differ significantly be- and stable isotope analyses of otolith material to identify tween species, suggesting they experience comparable interspecific differences in habitat and resource use thermal regimes. However, δ13C varied substantially, among juveniles of two sympatric and morphologically with the otoliths of A. goreensis depleted in 13Crelative indistinct bonefishes, A. goreensis and A. vulpes in to A. vulpes by approximately 1‰, potentially signify- littoral zones of The Bahamas. Both species occurred ing a greater reliance on pelagic carbon sources by the in similar water temperatures; however, A. goreensis former, in agreement with observed distinctions in hab- juveniles occupied habitats characterized by greater itat use. In linear models, otolith δ13C was negatively wave-driven flow velocities and closer proximity to correlated with ambient flow velocity and positively coral reefs than A. vulpes. Likewise, A. goreensis was related to distance from coral reef habitats, and these present across a broader range of flow environments and relationships did not vary across species. After account- sampling stations than A. vulpes,whichwastypically ing for the effects of these variables, species-specific confined to sheltered, low-flow habitats. The results of differences in otolith δ13C remained, indicating that stable isotope analyses were consistent with the species’ other unknown factors contributed to the observed dis- relationships with environmental parameters, providing parities. -

Inventory and Atlas of Corals and Coral Reefs, with Emphasis on Deep-Water Coral Reefs from the U
Inventory and Atlas of Corals and Coral Reefs, with Emphasis on Deep-Water Coral Reefs from the U. S. Caribbean EEZ Jorge R. García Sais SEDAR26-RD-02 FINAL REPORT Inventory and Atlas of Corals and Coral Reefs, with Emphasis on Deep-Water Coral Reefs from the U. S. Caribbean EEZ Submitted to the: Caribbean Fishery Management Council San Juan, Puerto Rico By: Dr. Jorge R. García Sais dba Reef Surveys P. O. Box 3015;Lajas, P. R. 00667 [email protected] December, 2005 i Table of Contents Page I. Executive Summary 1 II. Introduction 4 III. Study Objectives 7 IV. Methods 8 A. Recuperation of Historical Data 8 B. Atlas map of deep reefs of PR and the USVI 11 C. Field Study at Isla Desecheo, PR 12 1. Sessile-Benthic Communities 12 2. Fishes and Motile Megabenthic Invertebrates 13 3. Statistical Analyses 15 V. Results and Discussion 15 A. Literature Review 15 1. Historical Overview 15 2. Recent Investigations 22 B. Geographical Distribution and Physical Characteristics 36 of Deep Reef Systems of Puerto Rico and the U. S. Virgin Islands C. Taxonomic Characterization of Sessile-Benthic 49 Communities Associated With Deep Sea Habitats of Puerto Rico and the U. S. Virgin Islands 1. Benthic Algae 49 2. Sponges (Phylum Porifera) 53 3. Corals (Phylum Cnidaria: Scleractinia 57 and Antipatharia) 4. Gorgonians (Sub-Class Octocorallia 65 D. Taxonomic Characterization of Sessile-Benthic Communities 68 Associated with Deep Sea Habitats of Puerto Rico and the U. S. Virgin Islands 1. Echinoderms 68 2. Decapod Crustaceans 72 3. Mollusks 78 E. -

Zootaxa, a New Species of Ladyfish, of the Genus Elops
Zootaxa 2346: 29–41 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A new species of ladyfish, of the genus Elops (Elopiformes: Elopidae), from the western Atlantic Ocean RICHARD S. MCBRIDE1, CLAUDIA R. ROCHA2, RAMON RUIZ-CARUS1 & BRIAN W. BOWEN3 1Fish and Wildlife Research Institute, Florida Fish and Wildlife Conservation Commission, 100 8th Avenue SE, St. Petersburg, FL 33701 USA. E-mail: [email protected]; [email protected] 2University of Texas at Austin - Marine Science Institute, 750 Channel View Drive, Port Aransas, TX 78374 USA. E-mail: [email protected] 3Hawaii Institute of Marine Biology, P.O. Box 1346, Kaneohe, HI 96744 USA. E-mail: [email protected] Abstract This paper describes Elops smithi, n. sp., and designates a lectotype for E. saurus. These two species can be separated from the five other species of Elops by a combination of vertebrae and gillraker counts. Morphologically, they can be distinguished from each other only by myomere (larvae) or vertebrae (adults) counts. Elops smithi has 73–80 centra (total number of vertebrae), usually with 75–78 centra; E. saurus has 79–87 centra, usually with 81–85 centra. No other morphological character is known to separate E. smithi and E. saurus, but the sequence divergence in mtDNA cytochrome b (d = 0.023–0.029) between E. smithi and E. saurus is similar to or greater than that measured between recognized species of Elops in different ocean basins. Both species occur in the western Atlantic Ocean, principally allopatrically but with areas of sympatry, probably via larval dispersal of E. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste