IFB Forensics Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Multi-Class Determination of 64 Illicit Compounds in Dietary Supplements Using Liquid Chromatography–Tandem Mass Spectrometry
molecules Article Multi-Class Determination of 64 Illicit Compounds in Dietary Supplements Using Liquid Chromatography–Tandem Mass Spectrometry Dasom Shin, Hui-Seung Kang *, Hyungsoo Kim and Guiim Moon New Hazardous Substances Division, Department of Food Safety Evaluation, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Osong, Cheongju 28159, Korea; [email protected] (D.S.); [email protected] (H.K.); [email protected] (G.M.) * Correspondence: [email protected] Received: 11 August 2020; Accepted: 17 September 2020; Published: 24 September 2020 Abstract: In this work, liquid chromatography–tandem mass spectrometry (LC-MS/MS) method was developed and validated for screening and confirmation of 64 illicit compounds in dietary supplements. The target compounds were illegally used pharmaceutical drugs, prohibited compounds, and not authorized ingredients for different therapeutics (sexual enhancement, weight loss, muscular strengthening, and relaxing products). The validation procedure was performed to evaluate selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision according to the Association of Official Analytical Chemists guidelines. The linearity was >0.98 in the range of 1 1 0.5–200 µg L− . The LOQs were in the range 1–10 µg kg− for all target compounds. The accuracy (expressed as recovery) was 78.5–114%. The precision (expressed as the relative standard deviation) was below 9.15%. The developed method was applied for the determination of illicit compounds in dietary supplements collected from websites. As a result, the total detection rate was 13.5% (27 samples detected in 200 samples). The concentrations of detected samples ranged from 0.51 1 to 226 mg g− . -

Réglementation De La Pharmacie
R E C U E I L D E T E X T E S S U R L A P H A R M A C I E Mis à jour le 13 février 2017 par l’Inspection de la pharmacie P R É A M B U L E La réglementation relative à la pharmacie en vigueur en Nouvelle-Calédonie résulte de la coexistence des dispositions adoptées par la Nouvelle-Calédonie au titre de ses compétences en matières d’hygiène publique, de santé et de professions de la pharmacie1, et de celles adoptées par l’Etat au titre de ses compétences en matières de garanties des libertés publiques, de droit civil et de droit commercial2. Sur le contenu du recueil En 1954, la Nouvelle-Calédonie s’est vue étendre les articles L. 511 à L. 520 et L. 549 à L. 665 de l’ancien Livre V relatif à la Pharmacie du code de la santé publique métropolitain par la loi n° 54-418 du 15 avril 1954 étendant aux territoires d'outre-mer, au Togo et au Cameroun certaines dispositions du Code de la santé publique relatives à l'exercice de la pharmacie3, dont les modalités d’application ont été fixées par le décret modifié n° 55-1122 du 16 août 1955 fixant les modalités d'application de la loi n° 54-418 du 15 avril 1954 étendant aux territoires d'outre-mer, au Togo et au Cameroun certaines dispositions du code de la santé publique relatives à l'exercice de la pharmacie4. Depuis sont intervenues la loi- cadre Defferre5, la loi référendaire de 19886 et la loi organique n° 99-209 du 19 mars 1999 dont les apports ont eu pour résultat le transfert de ces articles de la compétence de l’Etat à la compétence de la Nouvelle-Calédonie, permettant à celle-ci de s’en approprier et de les modifier à sa guise par des délibérations du congrès de la Nouvelle-Calédonie7. -

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -

Meeting Materials)
California State Board of Pharmacy BUSINESS, CONSUMER SERVICES AND HOUSING AGENCY 1625 N. Market Blvd, N219, Sacramento, CA 95834 DEPARTMENT OF CONSUMER AFFAIRS Phone: (916) 574-7900 GOVERNOR EDMUND G. BROWN JR. Fax: (916) 574-8618 www.pharmacy.ca.gov ENFORCEMENT AND COMPOUNDING COMMITTEE REPORT April 18, 2017 Amy Gutierrez, PharmD, Licensee Member, Chair Allen Schaad, Licensee Member, Vice Chair Greg Lippe, Public Member Stan Weisser, Licensee Member Valerie Muñoz, Public Member Ricardo Sanchez, Public Member I. Call to Order, Establishment of Quorum, and General Announcements II. Public Comments on Items Not on the Agenda/Agenda Items for Future Meetings Note: The board may not discuss or take action on any matter raised during this public comment section that is not included on this agenda, except to decide whether to place the matter on the agenda of a future meeting. [Government Code sections 11125, 11125.7(a)] III. Enforcement Matters a. University of California, San Diego’s Pilot Program to Permit Patients to Access Medications from an Automated Drug Delivery System Not Immediately Adjacent to the Pharmacy Background At the April 2015 Board Meeting, the board approved an 18-month pilot study under the auspices of the University of California, San Diego (UCSD) School of Pharmacy involving use of an automated drug delivery system (ADDS) for prescription medication from which staff of Sharp Hospital in San Diego and their families, who opted in, could pick up their outpatient medications. Consultation would be provided via telephone before medication could be dispensed to a patient for first time fills. Since that time the committee has received quarterly updates on the study, including usage of the system. -

Review Memorandum
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k062165 B. Purpose for Submission: New device C. Measurand: Barbiturates D. Type of Test: Qualitative and semi-quantitative enzyme immunoassay E. Applicant: Ortho-Clinical Diagnostics, Inc. F. Proprietary and Established Names: VITROS Chemistry Products BARB Reagent VITROS Chemistry Products Calibrator 26 VITROS Chemistry Products FS Calibrator 1 VITROS Chemistry Products DAT Performance Verifiers I, II, III, IV and V G. Regulatory Information: 1. Regulation section: 21 CFR 862.3150, Barbiturates test system 21 CFR 862.3200, Clinical Toxicology Calibrator 21 CFR 862.3180, Clinical Toxicology Control 2. Classification: Class II, (reagent, calibrator) Class I, reserved (control) 3. Product code: DIS, DLJ and DIF 4. Panel: Toxicology (91) 1 H. Intended Use: 1. Intended use(s): See Indications for use. 2. Indication(s) for use: VITROS Chemistry Products BARB Reagent: For in vitro diagnostic use only. VITROS Chemistry Products BARB Reagent is used on VITROS 5,1 FS Chemistry Systems for the semi- quantitative or qualitative determination of barbiturates (BARB) in human urine using a cutoff of 200 ng/mL or 300 ng/mL. Measurements obtained with the VITROS BARB method are used in the diagnosis and treatment of barbiturates use or overdose. The VITROS Chemistry Products BARB assay is intended for use by professional laboratory personnel. It provides only a preliminary test result. A more specific alternative chemical method must be used to confirm a result with this assay. Gas Chromatograpy/Mass Spectrometry (GC/MS) is the preferred confirmatory method. Clinical consideration and professional judgment should be applied to any drug-of-abuse test result, particularly when evaluating a preliminary positive result. -

21 CFR Ch. II (4–1–10 Edition) § 1308.12
§ 1308.12 21 CFR Ch. II (4–1–10 Edition) Some trade and other names: N,N- whenever the existence of such salts, Diethyltryptamine; DET isomers, and salts of isomers is possible (18) Dimethyltryptamine ................................................. 7435 Some trade or other names: DMT within the specific chemical designa- (19) 5-methoxy-N,N-diisopropyltryptamine (other tion: name: 5-MeO-DIPT) ................................................... 7439 (1) gamma-hydroxybutyric acid (some other names in- (20) Ibogaine .................................................................. 7260 clude GHB; gamma-hydroxybutyrate; 4- Some trade and other names: 7-Ethyl- hydroxybutyrate; 4-hydroxybutanoic acid; sodium 6,6b,7,8,9,10,12,13-octahydro-2-methoxy-6,9- oxybate; sodium oxybutyrate) .................................... 2010 methano-5H-pyrido [1′, 2′:1,2] azepino [5,4-b] (2) Mecloqualone ........................................................... 2572 indole; Tabernanthe iboga (3) Methaqualone ........................................................... 2565 (21) Lysergic acid diethylamide ..................................... 7315 (22) Marihuana .............................................................. 7360 (f) Stimulants. Unless specifically ex- (23) Mescaline ............................................................... 7381 cepted or unless listed in another (24) Parahexyl—7374; some trade or other names: 3- schedule, any material, compound, Hexyl-1-hydroxy-7,8,9,10-tetrahydro-6,6,9-trimethyl- 6H-dibenzo[b,d]pyran; Synhexyl. mixture, -

Simultaneous Rapid HPLC Determination of Anticonvulsant Drugs in Plasma and Correlation with EMIT®
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 10, No. 1 Copyright© 1980, Institute for Clinical Science, Inc. Simultaneous Rapid HPLC Determination of Anticonvulsant Drugs in Plasma and Correlation with EMIT® RAYMOND S. RYDZEWSKI, M.S., RICHARD H. GADSDEN, P h .D.,* and CONSTANCE A. PHELPS, A.S.M.T. Division of Clinical Chemistry, Department of Laboratory Medicine, Medical University of South Carolina, Charleston, SC 29403 ABSTRACT A method is presented for measuring simultaneously five anti-convulsants (primidone, ethosuximide, phénobarbital, carbamazepine and diphenyl- hydantoin) in serum and plasma by high-performance liquid chroma tography (H PLC) usingalphenal (5 - allyl - 5 - phenyl - barbituric acid) as the internal standard. All five drugs are separated from each other and, in the case of primidone, from its metabolite, 2 - phenyl - 2 - ethyl - malondiamide. Total HPLC time for the separation is seven minutes. The chromatography is performed on a C-8 reverse phase column with a mobile phase consisting of acetonitrile/phosphate buffer (35/65) at 25°C. The eluted drugs are detected at 220 nm and quantitated from their peak heights relative to that of the internal standard. The lower limits of detection for each drug is 200 ng per ml for primidone, 1000 ng per ml for ethosuximide, 200 ng per ml for phéno barbital, 100 ng per ml for carbamazepine and 200 ng per ml for diphenyl- hydantoin. Analytical recoveries for the five drugs ranged from 97 to 107 percent. Correlation of results for 187 specimens by enzyme-immunoassay (EMIT) was 0.981 for primidone, 0.827 for ethosuximide, 0.975 for phéno barbital, 0.889 for carbamazepine and 0.990 for diphenylhydantoin. -

Author Index Page Numbers in Italics Refer to Bibliography
Author Index Page Numbers in italics refer to bibliography Aaron,T.H., Criep,L.H. 180. Adams,M.R., see Litchfield,J. Albert,A., Serjeant,E.P. 176, 207 T., Jr. 386, 409, 432, 441, 207 Aaron, T. H., see Criep, L. H. 465,496 Albert,J.R., see Lish,P.M. 398,427 Adamson,D.W., Barrett,P.A., 449, 465, 466, 495 Aarsen,P.N., Zeegers,A. 15, Billinghurst, J. W., Green, A. Albert, U., see Giertz, H. 444, 30 F .. Jones,T.S.G. 190,192, 489 Abbozzo, G., Genazzani, E., 207 Alberty,J. 387,425, 442, Donatelli, L. 562,570 Adamson, D. W., Barrett, P. A., 452--455,480 Abdel-Galil, A. A. M., Marshall, Billinghurst,J. W., Jones, T. Alberty, J., Huurrekorpi, L. P. B. 114, 122 S.G. 190,207 399, 425, 455, 480 Aborg, e.-H .. Novotny,J., Adamson, D. W., Billinghurst, Alberty,J., Schiede,M. 399, Uvniis,B. 86.90 J.W. 189,194,207 425,455,480 Aborg,e. H., Uvniis,B. 87, 90 Adashek, D., Grossman, M. I. Alberty, J., Takkunen, R. 467, Aborg, C.-H., see Uvniis,B. 58. 46,47,55 480 74. 75, 82, 84-87. 92 Adlerova, E., see Protiva, M. Albinus,M., Sewing.K.F. 2, Abram, L. E., see Cohen, M. B. 201, 212 3, 6, 8, 11, 21, 30, 264, 287, 421,427 Ado,A.D., Abrossimow,W.N. 463,480 Abrams,G., see Lear,E. 566, 453.480 Albrecht, I., see Cort, J. H. 579, 571 Ado, A. D., Ishimova, L. M., 597 Abramson, D. -

Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Pharmacology, Biochemistry and Behavior 83 (2006) 122–129 www.elsevier.com/locate/pharmbiochembeh Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats ⁎ W.E. Fantegrossi a,b, , A.W. Harrington b, C.L. Kiessel b, J.R. Eckler c, R.A. Rabin c, J.C. Winter c, A. Coop d, K.C. Rice e, J.H. Woods b a Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30322, United States b Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI, United States c Department of Pharmacology and Toxicology, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, United States d Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, United States e Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD Received 2 June 2005; received in revised form 7 December 2005; accepted 29 December 2005 Available online 3 February 2006 Abstract Few studies have examined the effects of 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) in vivo. In these studies, 5-MeO-DIPT was tested in a drug-elicited head twitch assay in mice where it was compared to the structurally similar hallucinogen N,N-dimethyltryptamine (N,N- DMT) and challenged with the selective serotonin (5-HT)2A antagonist M100907, and in a lysergic acid diethylamide (LSD) discrimination assay in rats where its subjective effects were challenged with M100907 or the 5-HT1A selective antagonist WAY-100635. Finally, the affinity of 5- MeO-DIPT for three distinct 5-HT receptors was determined in rat brain. -

Question of the Day Archives: Monday, December 5, 2016 Question: Calcium Oxalate Is a Widespread Toxin Found in Many Species of Plants
Question Of the Day Archives: Monday, December 5, 2016 Question: Calcium oxalate is a widespread toxin found in many species of plants. What is the needle shaped crystal containing calcium oxalate called and what is the compilation of these structures known as? Answer: The needle shaped plant-based crystals containing calcium oxalate are known as raphides. A compilation of raphides forms the structure known as an idioblast. (Lim CS et al. Atlas of select poisonous plants and mushrooms. 2016 Disease-a-Month 62(3):37-66) Friday, December 2, 2016 Question: Which oral chelating agent has been reported to cause transient increases in plasma ALT activity in some patients as well as rare instances of mucocutaneous skin reactions? Answer: Orally administered dimercaptosuccinic acid (DMSA) has been reported to cause transient increases in ALT activity as well as rare instances of mucocutaneous skin reactions. (Bradberry S et al. Use of oral dimercaptosuccinic acid (succimer) in adult patients with inorganic lead poisoning. 2009 Q J Med 102:721-732) Thursday, December 1, 2016 Question: What is Clioquinol and why was it withdrawn from the market during the 1970s? Answer: According to the cited reference, “Between the 1950s and 1970s Clioquinol was used to treat and prevent intestinal parasitic disease [intestinal amebiasis].” “In the early 1970s Clioquinol was withdrawn from the market as an oral agent due to an association with sub-acute myelo-optic neuropathy (SMON) in Japanese patients. SMON is a syndrome that involves sensory and motor disturbances in the lower limbs as well as visual changes that are due to symmetrical demyelination of the lateral and posterior funiculi of the spinal cord, optic nerve, and peripheral nerves. -
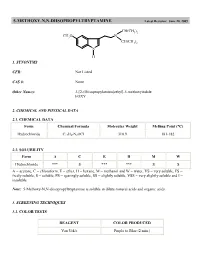
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4.