Mesothelium Contributes to Vascular Smooth Muscle and Mesenchyme During Lung Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mesocolon a Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization
ORIGINAL ARTICLE The Mesocolon A Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization Kevin Culligan, MRCS,∗ Stewart Walsh, FRCSEd,∗ Colum Dunne, PhD,∗ Michael Walsh, PhD,† Siobhan Ryan, MB,‡ Fabio Quondamatteo, MD,‡ Peter Dockery, PhD,§ and J. Calvin Coffey, FRCSI∗¶ uring fetal development, the dorsal mesentery suspends the en- Background: Colonic mobilization requires separation of mesocolon from tire gastrointestinal tract from the posterior abdominal wall. The underlying fascia. Despite the surgical importance of planes formed by these D mesocolon is the adult remnant of that part of the dorsal mesentery structures, no study has formally characterized their microscopic features. associated with the colon.1 In the adult human, the transverse and The aim of this study was to determine the histological and electron micro- lateral sigmoid portions of the mesocolon are mobile whereas the as- scopic appearance of mesocolon, fascia, and retroperitoneum, prior to and cending, descending, and medial sigmoid portions are nonmobile and after colonic mobilization. attached to underlying retroperitoneum.2–4 Classic anatomic teaching Methods: In 24 cadavers, samples were taken from right, transverse, de- maintains that the ascending and descending mesocolon “disappear” scending, and sigmoid mesocolon. In 12 cadavers, specimens were stained during embryogenesis.5,6 In keeping with this, the identification of a with hematoxylin and eosin (3 sections) or Masson trichrome (3 sections). In right or left mesocolon in the adult is frequently depicted as anoma- the second 12 cadavers, lymphatic channels were identified by staining im- lous rather than accepted as an anatomic norm.7 Accordingly, the munohistochemically for podoplanin. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

The Fingerprint Sourcebook
CHAPTER EMBRYOLOGY AND MORPHOLOGY OF FRICTION RIDGE SKIN Kasey Wertheim CONTENTS 3 3.1 Introduction 12 3.7 Pattern Formation 4 3.2 Embryology: Establishing 18 3.8 Genetics Uniqueness and Pattern Formation in the Friction Ridge Skin 5 3.3 Limb Development 21 3.9 Uniqueness: Developmental Noise 7 3.4 Differentiation of the Friction 22 3.10 Summary: Keys to Ridge Skin Uniqueness and Pattern Formation 8 3.5 Primary Ridge Formation 22 3.11 Reviewers 11 3.6 Secondary Ridge 24 3.12 References Formation 3–1 Embryology and Morphology of Friction Ridge Skin C H A P T E R 3 CHAPTER 3 EMBRYOLOGY AND 3.1 Introduction Friction ridge skin has unique features that persist from MORPHOLOGY OF before birth until decomposition after death. Upon contact with a surface, the unique features of friction ridge skin may leave an impression of corresponding unique details. Two FRICTION RIDGE SKIN impressions can be analyzed, compared, and evaluated, and if sufficient quality and quantity of detail is present (or Kasey Wertheim lacking) in a corresponding area of both impressions, a com- petent examiner can effect an individualization or exclusion (identify or exclude an individual). The analysis, comparison, evaluation, and verification (ACE-V) methodology, combined with the philosophy of quantitative–qualitative examinations, provide the framework for practical application of the friction ridge examination discipline. But at the heart of the disci- pline is the fundamental principle that allows for conclusive determinations: the source of the impression, friction ridge skin, is unique and persistent. Empirical data collected in the medical and forensic com- munities continues to validate the premises of uniqueness and persistence. -

ABDOMINOPELVIC CAVITY and PERITONEUM Dr
ABDOMINOPELVIC CAVITY AND PERITONEUM Dr. Milton M. Sholley SUGGESTED READING: Essential Clinical Anatomy 3 rd ed. (ECA): pp. 118 and 135141 Grant's Atlas Figures listed at the end of this syllabus. OBJECTIVES:Today's lectures are designed to explain the orientation of the abdominopelvic viscera, the peritoneal cavity, and the mesenteries. LECTURE OUTLINE PART 1 I. The abdominopelvic cavity contains the organs of the digestive system, except for the oral cavity, salivary glands, pharynx, and thoracic portion of the esophagus. It also contains major systemic blood vessels (aorta and inferior vena cava), parts of the urinary system, and parts of the reproductive system. A. The space within the abdominopelvic cavity is divided into two contiguous portions: 1. Abdominal portion that portion between the thoracic diaphragm and the pelvic brim a. The lower part of the abdominal portion is also known as the false pelvis, which is the part of the pelvis between the two iliac wings and above the pelvic brim. Sagittal section drawing Frontal section drawing 2. Pelvic portion that portion between the pelvic brim and the pelvic diaphragm a. The pelvic portion of the abdominopelvic cavity is also known as the true pelvis. B. Walls of the abdominopelvic cavity include: 1. The thoracic diaphragm (or just “diaphragm”) located superiorly and posterosuperiorly (recall the domeshape of the diaphragm) 2. The lower ribs located anterolaterally and posterolaterally 3. The posterior abdominal wall located posteriorly below the ribs and above the false pelvis and formed by the lumbar vertebrae along the posterior midline and by the quadratus lumborum and psoas major muscles on either side 4. -

Primary Retroperitoneal Mucinous Cystadenoma
Case Reports Primary retroperitoneal mucinous cystadenoma Malak S. Abedalthagafi, MD, Patrick G. Jackson, MD, Metin Ozdemirli, MD, PhD. rimary mucinous cystadenomas of the ABSTRACT Pretroperitoneum are extremely rare tumors. Although very rare cases were reported in men and children, these tumors are found exclusively in تتضمن أورام خلف الصفاق اﻷولي: السرطان الكيسي املخاطي، women.1-3 Like most retroperitoneal tumors, they can اﻷورام املخاطية ذات احلد الفاصل، اﻷورام النادرة واملتواجدة في cause symptoms through exertion of pressure or by النساء واملتضمنة احلزام املخاطي. وحيث أن خلف الصفاق اﻷولي .obstructing adjacent organs if they are large enough ﻻ يحتوي على ظاهرة مخاطية، تبقى نظرية حدوث هذه اﻷورام .They have potential for malignant transformation غير معروفة. نستنتج أن حدوث هذه اﻷورام قد يأتي من اﻷورام There is no unanimous opinion on the genesis of املسخية، أو من املبايض الزائدة، أو من التحول املخاطي للطبقة these tumors and due to their extreme rarity, their املتوسطة خللف الصفاق. نستعرض في هذا التقرير حالة للخدام histogenesis, biological behavior, and their optimal املخاطي خلف الصفاق اﻷولي ملريضة تبلغ من العمر 44 ًعاما، والتي ,management remains at a speculative level. In this paper حضرت بسبب ورم بطني. بعد استئصال الورم بجراحة املنظار we present a case of primary retroperitoneal mucinous لم يكن هناك أية أثر لعودة الورم بعد 16 شهرا.ً الشكل املجهري cystadenoma and review the clinicopathological features, therapeutic options, and outcome in respect والتحليل للصبغات النسيجية يدعم فرضية التحول املخاطي لطبقة to the cases reported in the literature. The morphologic خلف الصفاق املتوسطة واملسبوقة بتكوين كيسي اشتمالي والتي and immunohistochemical analysis observed in this تؤدي إلى حدوث أورام خلف الصفاق املخاطية. -

Coordination of Endothelial Cell Positioning and Fate Specification By
ARTICLE https://doi.org/10.1038/s41467-021-24414-z OPEN Coordination of endothelial cell positioning and fate specification by the epicardium Pearl Quijada 1,8, Michael A. Trembley1, Adwiteeya Misra1,2, Jacquelyn A. Myers 3,4, Cameron D. Baker 3,4, Marta Pérez-Hernández 5, Jason R. Myers3,4, Ronald A. Dirkx Jr.1, ✉ Ethan David Cohen6, Mario Delmar5, John M. Ashton 3,4 & Eric M. Small 1,2,7 The organization of an integrated coronary vasculature requires the specification of immature 1234567890():,; endothelial cells (ECs) into arterial and venous fates based on their localization within the heart. It remains unclear how spatial information controls EC identity and behavior. Here we use single-cell RNA sequencing at key developmental timepoints to interrogate cellular contributions to coronary vessel patterning and maturation. We perform transcriptional profiling to define a heterogenous population of epicardium-derived cells (EPDCs) that express unique chemokine signatures. We identify a population of Slit2+ EPDCs that emerge following epithelial-to- mesenchymal transition (EMT), which we term vascular guidepost cells. We show that the expression of guidepost-derived chemokines such as Slit2 are induced in epicardial cells undergoing EMT, while mesothelium-derived chemokines are silenced. We demonstrate that epicardium-specific deletion of myocardin-related transcription factors in mouse embryos disrupts the expression of key guidance cues and alters EPDC-EC signaling, leading to the persistence of an immature angiogenic EC identity and inappropriate accumulation of ECs on the epicardial surface. Our study suggests that EC pathfinding and fate specification is controlled by a common mechanism and guided by paracrine signaling from EPDCs linking epicardial EMT to EC localization and fate specification in the developing heart. -

Development of the Skin and Its Derivatives
Development of the skin and its derivatives Resources: http://php.med.unsw.edu.au/embryology/ Larsen’s Human Embryology – Chapter 7 The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam – School of Medical Sciences, UNSW Wallace Wurth Building Room 234 – [email protected] Lecture overview Skin function and anatomy Skin origins Development of the overlying epidermis Development of epidermal appendages: Hair follicles Glands Nails Teeth Development of melanocytes Development of the Dermis Resources: http://php.med.unsw.edu.au/embryology/ Larsen’s Human Embryology – Chapter 7 The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam – School of Medical Sciences, UNSW Wallace Wurth Building Room 234 – [email protected] Skin Function and Anatomy Largest organ of our body Protects inner body from outside world (pathogens, water, sun) Thermoregulation Diverse: thick vs thin skin, scalp skin vs face skin, etc Consists of: - Overlying epidermis - Epidermal appendages: - Hair follicles, - Glands: sebaceous, sweat, apocrine, mammary - Nails - Teeth - Melanocytes - (Merkel Cells - Langerhans cells) - Dermis - Hypodermis Skin origins Trilaminar embryo Ectoderm (Neural crest) brain, spinal cord, eyes, peripheral nervous system epidermis of skin and associated structures, melanocytes, cranial connective tissues (dermis) Mesoderm musculo-skeletal system, limbs connective tissue of skin and organs urogenital system, heart, blood cells Endoderm epithelial linings of gastrointestinal and respiratory tracts Ectoderm -
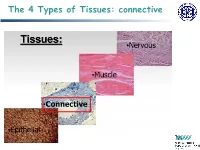
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

Development of the Serosal Mesothelium
J. Dev. Biol. 2013, 1, 64-81; doi:10.3390/jdb1020064 OPEN ACCESS Journal of Developmental Biology ISSN 2221-3759 www.mdpi.com/journal/jdb Review Development of the Serosal Mesothelium Nichelle I. Winters and David M. Bader * Department of Medicine, Vanderbilt University, 2220 Pierce Ave Nashville, TN 37232, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-615-936-1976; Fax: +1-615-936-3527. Received: 3 May 2013; in revised form: 13 June 2013 / Accepted: 19 June 2013 / Published: 26 June 2013 Abstract: Mesothelia in the adult vertebrate are the simple squamous epithelia covering all coelomic organs and body cavities. Until recently, analysis of the generation and differentiative potential of mesothelia in organogenesis has largely focused on development of visceral mesothelium of the heart; the epicardium and its progenitor, the proepicardium. Here, we review emerging data on the development and differentiation of serosal mesothelium, the covering of the gastrointestinal tract. This literature demonstrates that serosal mesothelium is generated through a completely different mechanism than that seen in the heart suggesting that commitment of progenitors to this cell lineage does not follow a common pathway. The differentiative potential of serosal mesothelium is also discussed in comparison to that observed for progeny of the proepicardium/epicardium. In our review of the literature, we point out gaps in our understanding of serosal mesothelial development and that of mesothelial development as a whole. Keywords: mesothelium; proepicardium; epicardium; intestine; heart 1. Mesothelia: Broad Definition Mesothelia are simple squamous epithelia that line coelomic cavities and organs and form the mesenteries. -

The Role of the Mesenchyme in 1. Patterns of Mesenchymal Cell
THE AMERICAN JOURNAL OF ANATOMY 188:121-132 (1990) The Role of the Mesenchyme in Mouse Neural Fold Elevation 1. Patterns of Mesenchymal Cell Distribution and Proliferation in Embryos Developing In Vitro JOYCE MORRIS-WIMAN AND LINDA L. BRINKLEY Department of Anatomy & Cell Biology, University of Michigan Medical School, Ann Arbor, Michigan 48109 ABSTRACT Using the computer-assisted a decrease in the density of cells residing in the central method of smoothed spatial averaging, spatial region of the mesenchyme and with a decrease in the and temporal patterns of cell distribution and mi- concentration of hyaluronate (HA) in this region (Mor- totic activity were analyzed in the cranial mesen- ris-Wiman and Brinkley, 1989). The decrease observed chyme underlying the mesencephalic neural folds in mesenchymal cell density during elevation could re- sult from a decrease in mitotic rate, from an increase in of mouse embryos maintained in roller tube cul- cell death, or from the expanion of the extracellular ture. Total cell density increased in central and matrix of the cranial mesenchyme displacing cells from medial mesenchymal regions after 12 hr in cul- this region. The glycosaminoglycan hyaluronate is the ture, decreased after 18 hr, and showed a further major component of this extracellular matrix (Solursh decrease after 24 hr when the neural folds of the and Morriss, 1977; Morriss and Solursh, 1978a,b;Hei- embryos had elevated, converged, and were fus- fetz et al., 1980). In biological and experimental sys- ing or fused. Mitotic activity, as measured by the tems, it is capable of a high degree of hydration with a ratio of 3H-thymidine-labeled cells to unlabeled concomitant increase in volume (Comper and Laurent, cells, was highest in the central mesenchyme at all 1978). -

Role of Extracellular Matrix in Regulating Embryonic Epithelial-Mesenchymal Transition
BioMol Concepts, Vol. 3 (2012), pp. 333–344 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/bmc-2011-0065 Review Role of extracellular matrix in regulating embryonic epithelial-mesenchymal transition Francesca Zito considerable amount of data about its basic mechanisms. Istituto di Biomedicina e Immunologia Molecolare From a clinical viewpoint, the results obtained so far offer ‘ Alberto Monroy ’ , Consiglio Nazionale delle Ricerche, a great promise for the development of new therapies. At Via Ugo La Malfa 153, I-90146 Palermo , Italy the same time, they advance our knowledge about essential developmental processes. e-mail: [email protected] Although EMT is a widely studied topic, it is likely that not all aspects of this process have been elucidated so far. Considerable knowledge has been accumulated regarding cell Abstract morphological changes, and the regulation of several genes and various signaling pathways have been partially under- Embryogenesis and morphogenesis are characterized by stood. Still, little is known about the role of the extracellular complex cell rearrangements and movements which require matrix (ECM) as a cell substrate during EMT, and cell motility appropriate interactions of cells with the surrounding extracel- is probably the least understood aspect of the whole process. lular matrix (ECM) by means of specifi c membrane receptors. Many events in developing embryo are associated with Interest in the identifi cation and purifi cation of ECM com- cell migration, which consists of the coordinated move- ponents, as well as in conducting functional studies of them, ments of several different cells over short or long distances including their ligands and other molecules involved in cell- throughout the embryo. -

Metamorphic Changes of Dermis in Skin of Frog Larvae Exposed to Thyroxine’
DEVELOPMENTAL BIOLOGY, 7, 244-254 (1963) Metamorphic Changes of Dermis in Skin of Frog Larvae Exposed to Thyroxine’ NORMAN E. KEMP Department of Zoology, The University of Michigan, Ann Arbor, Michigan Accepted October 22, 1962 INTRODUCTION Skin in the tadpole of anuran larvae prior to metamorphosis is composed of two layers of epidermal cells (Leeson and Threadgold, 1961; Chapman and Dawson, 1961; Edds and Sweeny, 1960, 1962), a laminated basement lamella, and a layer of dermal mesenchyme cells apposed to the underside of the basement lamella. An adepidermal space and membrane (Salpeter and Singer, 1959, 1960) separate epi- dermis from the dermal basement lamella. Weiss and Ferris (1954) first observed that mesenchyme cells migrate into the basement lamella as metamorphosis begins. Invasion of the lamella has been reported to begin about Taylor-Kollros stage XI or XII (Kemp, 1961b) and to proceed until mesenchyme cells move entirely through the lamella and cause it to become detached from the adepidermal mem- brane at stages XV-XVI. Subsequently a population of cells consti- tuting the stratum spongiosum develops beneath the adepiderma1 membrane and the basement lamella becomes the deeper-lying stra- tum compacturn or “dermal lamella” of the adult dermis. New poly- merization of collagenous fibers and ground substance results in deposition of the adult basement membrane between the adepidermal membrane and the stratum spongiosum. Generalized metamorphic changes can be induced in amphibian larvae by exposing them to thyroxine or other thyroid hormones (Kollros, 1959; Pitt-Rivers and Tata, 1959). Local metamorphic changes can be induced by implanting pellets of thyroxine mixed with ‘This investigation was supported by grants from the United States Public Health Service (RG 5867-C>), the National Science Foundation (G-19447), and the Michigan Memorial-Phoenix Project (no.