Mesothelium and Malignant Mesothelioma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malignant Pleural Mesothelioma
CLINICAL PRACTICE GUIDELINE LU-009 Version 2 MALIGNANT PLEURAL MESOTHELIOMA Effective Date: December, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Malignancies Tumour Team synthesis of currently accepted approaches to management, derived from a review of relevant scientific literature. Clinicians applying these guidelines should, in consultation with the patient, use independent medical judgment in the context of individual clinical circumstances to direct care. CLINICAL PRACTICE GUIDELINE LU-009 Version 2 BACKGROUND Mesothelioma is a rare asbestos-related tumour that arises from mesenchymal cells that are found in the lining of the pleural cavity (Malignant Pleural Mesothelioma; MPM) in 70 to 90 percent of cases, and the peritoneal cavity in 10 to 30 percent of cases.1, 2 Due to the long latency period between exposure and disease, which has been reported to be between 30 and 50 years, most cases of mesothelioma being diagnosed today are the result of asbestos exposure in the 1960s and 1970s.3 Although safety measures for the use of asbestos were adopted in most countries several decades ago, the incidence rates, which are highly age-specific, are still rising, and are expected to peak over the next two decades.4-6 In Canada, the number of men diagnosed with mesothelioma has been steadily increasing over the past 20 years: there were 153 cases reported in 1984 versus 344 cases reported in 2003.3 Mesothelioma is less common in women: there were 78 Canadian women diagnosed with mesothelioma in 2003.3 In the United States, the peak mesothelioma incidence occurred in the early to mid-1990s and has possibly started to decline since then. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

Nasal Cavity Trachea Right Main (Primary) Bronchus Left Main (Primary) Bronchus Nostril Oral Cavity Pharynx Larynx Right Lung
Nasal cavity Oral cavity Nostril Pharynx Larynx Trachea Left main Right main (primary) (primary) bronchus bronchus Left lung Right lung Diaphragm © 2018 Pearson Education, Inc. 1 Cribriform plate of ethmoid bone Sphenoidal sinus Frontal sinus Posterior nasal aperture Nasal cavity • Nasal conchae (superior, Nasopharynx middle, and inferior) • Pharyngeal tonsil • Nasal meatuses (superior, middle, and inferior) • Opening of pharyngotympanic • Nasal vestibule tube • Nostril • Uvula Hard palate Oropharynx • Palatine tonsil Soft palate • Lingual tonsil Tongue Laryngopharynx Hyoid bone Larynx Esophagus • Epiglottis • Thyroid cartilage Trachea • Vocal fold • Cricoid cartilage (b) Detailed anatomy of the upper respiratory tract © 2018 Pearson Education, Inc. 2 Pharynx • Nasopharynx • Oropharynx • Laryngopharynx (a) Regions of the pharynx © 2018 Pearson Education, Inc. 3 Posterior Mucosa Esophagus Submucosa Trachealis Lumen of Seromucous muscle trachea gland in submucosa Hyaline cartilage Adventitia (a) Anterior © 2018 Pearson Education, Inc. 4 Intercostal muscle Rib Parietal pleura Lung Pleural cavity Trachea Visceral pleura Thymus Apex of lung Left superior lobe Right superior lobe Oblique Horizontal fissure fissure Right middle lobe Left inferior lobe Oblique fissure Right inferior lobe Heart (in pericardial cavity of mediastinum) Diaphragm Base of lung (a) Anterior view. The lungs flank mediastinal structures laterally. © 2018 Pearson Education, Inc. 5 Posterior Vertebra Esophagus (in posterior mediastinum) Root of lung at hilum Right lung • Left main bronchus Parietal pleura • Left pulmonary artery • Left pulmonary vein Visceral pleura Pleural cavity Left lung Thoracic wall Pulmonary trunk Pericardial membranes Heart (in mediastinum) Sternum Anterior mediastinum Anterior (b) Transverse section through the thorax, viewed from above © 2018 Pearson Education, Inc. 6 Alveolar duct Alveoli Respiratory bronchioles Alveolar duct Terminal bronchiole Alveolar sac (a) Diagrammatic view of respiratory bronchioles, alveolar ducts, and alveoli © 2018 Pearson Education, Inc. -
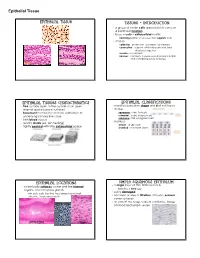
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

The Mesocolon a Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization
ORIGINAL ARTICLE The Mesocolon A Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization Kevin Culligan, MRCS,∗ Stewart Walsh, FRCSEd,∗ Colum Dunne, PhD,∗ Michael Walsh, PhD,† Siobhan Ryan, MB,‡ Fabio Quondamatteo, MD,‡ Peter Dockery, PhD,§ and J. Calvin Coffey, FRCSI∗¶ uring fetal development, the dorsal mesentery suspends the en- Background: Colonic mobilization requires separation of mesocolon from tire gastrointestinal tract from the posterior abdominal wall. The underlying fascia. Despite the surgical importance of planes formed by these D mesocolon is the adult remnant of that part of the dorsal mesentery structures, no study has formally characterized their microscopic features. associated with the colon.1 In the adult human, the transverse and The aim of this study was to determine the histological and electron micro- lateral sigmoid portions of the mesocolon are mobile whereas the as- scopic appearance of mesocolon, fascia, and retroperitoneum, prior to and cending, descending, and medial sigmoid portions are nonmobile and after colonic mobilization. attached to underlying retroperitoneum.2–4 Classic anatomic teaching Methods: In 24 cadavers, samples were taken from right, transverse, de- maintains that the ascending and descending mesocolon “disappear” scending, and sigmoid mesocolon. In 12 cadavers, specimens were stained during embryogenesis.5,6 In keeping with this, the identification of a with hematoxylin and eosin (3 sections) or Masson trichrome (3 sections). In right or left mesocolon in the adult is frequently depicted as anoma- the second 12 cadavers, lymphatic channels were identified by staining im- lous rather than accepted as an anatomic norm.7 Accordingly, the munohistochemically for podoplanin. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

ABDOMINOPELVIC CAVITY and PERITONEUM Dr
ABDOMINOPELVIC CAVITY AND PERITONEUM Dr. Milton M. Sholley SUGGESTED READING: Essential Clinical Anatomy 3 rd ed. (ECA): pp. 118 and 135141 Grant's Atlas Figures listed at the end of this syllabus. OBJECTIVES:Today's lectures are designed to explain the orientation of the abdominopelvic viscera, the peritoneal cavity, and the mesenteries. LECTURE OUTLINE PART 1 I. The abdominopelvic cavity contains the organs of the digestive system, except for the oral cavity, salivary glands, pharynx, and thoracic portion of the esophagus. It also contains major systemic blood vessels (aorta and inferior vena cava), parts of the urinary system, and parts of the reproductive system. A. The space within the abdominopelvic cavity is divided into two contiguous portions: 1. Abdominal portion that portion between the thoracic diaphragm and the pelvic brim a. The lower part of the abdominal portion is also known as the false pelvis, which is the part of the pelvis between the two iliac wings and above the pelvic brim. Sagittal section drawing Frontal section drawing 2. Pelvic portion that portion between the pelvic brim and the pelvic diaphragm a. The pelvic portion of the abdominopelvic cavity is also known as the true pelvis. B. Walls of the abdominopelvic cavity include: 1. The thoracic diaphragm (or just “diaphragm”) located superiorly and posterosuperiorly (recall the domeshape of the diaphragm) 2. The lower ribs located anterolaterally and posterolaterally 3. The posterior abdominal wall located posteriorly below the ribs and above the false pelvis and formed by the lumbar vertebrae along the posterior midline and by the quadratus lumborum and psoas major muscles on either side 4. -

Primary Retroperitoneal Mucinous Cystadenoma
Case Reports Primary retroperitoneal mucinous cystadenoma Malak S. Abedalthagafi, MD, Patrick G. Jackson, MD, Metin Ozdemirli, MD, PhD. rimary mucinous cystadenomas of the ABSTRACT Pretroperitoneum are extremely rare tumors. Although very rare cases were reported in men and children, these tumors are found exclusively in تتضمن أورام خلف الصفاق اﻷولي: السرطان الكيسي املخاطي، women.1-3 Like most retroperitoneal tumors, they can اﻷورام املخاطية ذات احلد الفاصل، اﻷورام النادرة واملتواجدة في cause symptoms through exertion of pressure or by النساء واملتضمنة احلزام املخاطي. وحيث أن خلف الصفاق اﻷولي .obstructing adjacent organs if they are large enough ﻻ يحتوي على ظاهرة مخاطية، تبقى نظرية حدوث هذه اﻷورام .They have potential for malignant transformation غير معروفة. نستنتج أن حدوث هذه اﻷورام قد يأتي من اﻷورام There is no unanimous opinion on the genesis of املسخية، أو من املبايض الزائدة، أو من التحول املخاطي للطبقة these tumors and due to their extreme rarity, their املتوسطة خللف الصفاق. نستعرض في هذا التقرير حالة للخدام histogenesis, biological behavior, and their optimal املخاطي خلف الصفاق اﻷولي ملريضة تبلغ من العمر 44 ًعاما، والتي ,management remains at a speculative level. In this paper حضرت بسبب ورم بطني. بعد استئصال الورم بجراحة املنظار we present a case of primary retroperitoneal mucinous لم يكن هناك أية أثر لعودة الورم بعد 16 شهرا.ً الشكل املجهري cystadenoma and review the clinicopathological features, therapeutic options, and outcome in respect والتحليل للصبغات النسيجية يدعم فرضية التحول املخاطي لطبقة to the cases reported in the literature. The morphologic خلف الصفاق املتوسطة واملسبوقة بتكوين كيسي اشتمالي والتي and immunohistochemical analysis observed in this تؤدي إلى حدوث أورام خلف الصفاق املخاطية. -

Coordination of Endothelial Cell Positioning and Fate Specification By
ARTICLE https://doi.org/10.1038/s41467-021-24414-z OPEN Coordination of endothelial cell positioning and fate specification by the epicardium Pearl Quijada 1,8, Michael A. Trembley1, Adwiteeya Misra1,2, Jacquelyn A. Myers 3,4, Cameron D. Baker 3,4, Marta Pérez-Hernández 5, Jason R. Myers3,4, Ronald A. Dirkx Jr.1, ✉ Ethan David Cohen6, Mario Delmar5, John M. Ashton 3,4 & Eric M. Small 1,2,7 The organization of an integrated coronary vasculature requires the specification of immature 1234567890():,; endothelial cells (ECs) into arterial and venous fates based on their localization within the heart. It remains unclear how spatial information controls EC identity and behavior. Here we use single-cell RNA sequencing at key developmental timepoints to interrogate cellular contributions to coronary vessel patterning and maturation. We perform transcriptional profiling to define a heterogenous population of epicardium-derived cells (EPDCs) that express unique chemokine signatures. We identify a population of Slit2+ EPDCs that emerge following epithelial-to- mesenchymal transition (EMT), which we term vascular guidepost cells. We show that the expression of guidepost-derived chemokines such as Slit2 are induced in epicardial cells undergoing EMT, while mesothelium-derived chemokines are silenced. We demonstrate that epicardium-specific deletion of myocardin-related transcription factors in mouse embryos disrupts the expression of key guidance cues and alters EPDC-EC signaling, leading to the persistence of an immature angiogenic EC identity and inappropriate accumulation of ECs on the epicardial surface. Our study suggests that EC pathfinding and fate specification is controlled by a common mechanism and guided by paracrine signaling from EPDCs linking epicardial EMT to EC localization and fate specification in the developing heart. -

Development of the Serosal Mesothelium
J. Dev. Biol. 2013, 1, 64-81; doi:10.3390/jdb1020064 OPEN ACCESS Journal of Developmental Biology ISSN 2221-3759 www.mdpi.com/journal/jdb Review Development of the Serosal Mesothelium Nichelle I. Winters and David M. Bader * Department of Medicine, Vanderbilt University, 2220 Pierce Ave Nashville, TN 37232, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-615-936-1976; Fax: +1-615-936-3527. Received: 3 May 2013; in revised form: 13 June 2013 / Accepted: 19 June 2013 / Published: 26 June 2013 Abstract: Mesothelia in the adult vertebrate are the simple squamous epithelia covering all coelomic organs and body cavities. Until recently, analysis of the generation and differentiative potential of mesothelia in organogenesis has largely focused on development of visceral mesothelium of the heart; the epicardium and its progenitor, the proepicardium. Here, we review emerging data on the development and differentiation of serosal mesothelium, the covering of the gastrointestinal tract. This literature demonstrates that serosal mesothelium is generated through a completely different mechanism than that seen in the heart suggesting that commitment of progenitors to this cell lineage does not follow a common pathway. The differentiative potential of serosal mesothelium is also discussed in comparison to that observed for progeny of the proepicardium/epicardium. In our review of the literature, we point out gaps in our understanding of serosal mesothelial development and that of mesothelial development as a whole. Keywords: mesothelium; proepicardium; epicardium; intestine; heart 1. Mesothelia: Broad Definition Mesothelia are simple squamous epithelia that line coelomic cavities and organs and form the mesenteries. -

Squamous Epithelium in the Human Thyroid Gland
J Clin Pathol: first published as 10.1136/jcp.19.4.384 on 1 July 1966. Downloaded from J. clin. Path. (1966), 19, 384. Squamous epithelium in the human thyroid gland J. N. HARCOURT-WEBSTER1 From the Department ofPathology, University ofEdinburgh SYNOPSIS Four cases are reported in each of which squamous epithelium was an incidental finding in surgically excised thyroid gland tissue. The occasional thyroid cyst lined throughout by squamous cells probably represents a persistent ultimo-branchial body, but the evidence indicates that the usual source of such cells in this gland is metaplasia of the follicular epithelium. An explanation is offered for the infrequency of this transformation in the thyroid, despite the frequent occurrence of the changes which predispose to epithelial metaplasia at other sites. There is no evidence to suggest that squamous cells arising in this gland by either of these means have any sinister significance. Squamous epithelium was first described in a human iodine uptake studies were normal but serological anti- thyroid gland by Nicholson (1922); he attributed body tests were positive (ADT: +ve on second day; this finding to metaplasia of the follicular epithelium TCH: 1/250,000; CFT: 1/250). The provisional diagnosis was Hashimoto's disease; a biopsy was taken from the induced by severe chronic inflammatory and fibrotic right lobe with a wedge resection of the isthmus. changes in the gland. Epithelial metaplasia occurs in Histology Sections of the rock-hard, pale fawn tissue response to altered function or at least as the result show abundant, interwoven, thick bands of hyaline col- of altered environment (Boyd, 1961).