Development of the Serosal Mesothelium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

A Comparative Study of the Ultrastructure of Microvilli in the Epithelium of Small and Large Intestine of Mice
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central A COMPARATIVE STUDY OF THE ULTRASTRUCTURE OF MICROVILLI IN THE EPITHELIUM OF SMALL AND LARGE INTESTINE OF MICE T. M. MUKHERJEE and A. WYNN WILLIAMS From the Electron Microscope Laboratory, the Departlnent of Pathology, the University of Otago Medical School, Dunedin, New Zealand ABSTRACT A comparative analysis of the fine structure of the microvilli on jejunal and colonic epi- thelial cells of the mouse intestine has been made. The microvilli in these two locations demonstrate a remarkably similar fine structure with respect to the thickness of the plasma membrane, the extent of the filament-free zone, and the characteristics of the microfila- ments situated within the microvillous core. Some of the core microfilaments appear to continue across the plasma membrane limiting the tip of the microvillus. The main differ- ence between the microvilli of small intestine and colon is in the extent and organization of the surface coat. In the small intestine, in addition to the commonly observed thin surface "fuzz," occasional areas of the jejunal villus show a more conspicuous surface coat covering the tips of the microvilli. Evidence has been put forward which indicates that the surface coat is an integral part of the epithelial cells. In contrast to the jejunal epithelium, the colonic epithelium is endowed with a thicker surface coat. Variations in the organization of the surface coat at different levels of the colonic crypts have also been noted. The func- tional significance of these variations in the surface coat is discussed. -

BODY CAVITIES and MESENTERY
73: BODY CAVITIES and MESENTERY We've already mentioned that all the organs in the body are wrapped in "bags" made of thin layers of connective tissue. These bags are often inside of other bags, or even inside of several bags. The largest bags define areas that we call body cavities. There are three main cavities: the thoracic cavity, the abdominal cavity and the pelvic cavity. The thoracic cavity is subdivided into three smaller cavities: the pleural cavity (containing the lungs), the mediastinum(in the middle), and the pericardial cavity (containing the heart). The pleural cavity is easy to understand because it simply contains the lungs. The pericardial cavity contains not only the heart itself, but the large blood vessels that come out of it, such as the aorta. The pericardial cavity is inside of the third cavity, the mediastinum. ("Media" means "middle" and "stinum" can refer to the "sternum," which is the bone that runs down the center of the ribcage.) The mediastinum contains not only the pericardial cavity but also part of the esophagus and trachea, the thymus (remember this organ from module 2 on the immune system?), and quite a few nerves and lymph nodes. The thin layers of connective tissues that surround these cavities are made primarily of collagen and elastin (produced by fibroblast cells) but they also contain some very tiny nerves and blood vessels, as well as cells that make serous fluid. As we've seen in the past few lessons, the diaphragm separates the thoracic cavity from the abdominal cavity. The abdominal cavity contains the stomach, the spleen, the tail of the pancreas, the last half of the duodenum, the small intestines, most of the large intestines, and the mesentery (thin layers of connective tissue that anchor the intestines to the back wall of the abdominal cavity). -

Nucleus Cytoplasm Plasma Membrane (A) Generalized Animal
Nucleus Cytoplasm Plasma membrane (a) Generalized animal cell © 2018 Pearson Education, Inc. 1 Nuclear envelope Chromatin Nucleus Nucleolus Nuclear pores (b) Nucleus 2 Extracellular fluid Glycoprotein Glycolipid (watery environment) Cholesterol Sugar group Polar heads of phospholipid molecules Bimolecular lipid layer containing proteins Channel Nonpolar tails of Proteins Filaments of phospholipid molecules cytoskeleton Cytoplasm (watery environment) 3 Microvilli Tight (impermeable) junction Desmosome (anchoring junction) Plasma membranes of adjacent cells Connexon Underlying Extracellular Gap basement space between (communicating) membrane cells junction 4 Chromatin Nuclear envelope Nucleolus Nucleus Plasma Smooth endoplasmic membrane reticulum Cytosol Lysosome Mitochondrion Rough endoplasmic reticulum Centrioles Ribosomes Golgi apparatus Secretion being released Microtubule from cell by exocytosis Peroxisome Intermediate filaments 5 Ribosome mRNA 1 As the protein is synthesized on the ribosome, Rough ER it migrates into the rough ER tunnel system. 2 1 3 2 In the tunnel, the protein folds into its functional shape. Short sugar chains may be attached to the protein (forming a glycoprotein). Protein 3 The protein is packaged in a tiny membranous sac called a transport vesicle. Transport 4 vesicle buds off 4 The transport vesicle buds from the rough ER and travels to the Golgi apparatus for further processing. Protein inside transport vesicle © 2018 Pearson Education, Inc. 6 Rough ER Tunnels Proteins in tunnels Membrane Lysosome fuses with ingested substances. Transport vesicle Golgi vesicle containing digestive enzymes becomes a lysosome. Pathway 3 Pathway 2 Golgi vesicle containing Golgi membrane components apparatus Secretory vesicles fuses with the plasma Pathway 1 membrane and is Proteins incorporated into it. Golgi vesicle containing proteins to be secreted Plasma membrane becomes a secretory Secretion by vesicle. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -
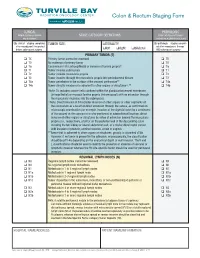
Colon & Rectum Staging Form
Colon & Rectum Staging Form CLINICAL PATHOLOGIC Extent of disease before STAGE CATEGORY DEFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical – staging completed TUMOR SIZE: LATERALITY: y pathologic – staging complet- after neoadjuvant therapy but ed after neoadjuvant therapy before subsequent surgery left right bilateral AND subsequent surgery PRIMARY TUMOR (T) TX Primary tumor cannot be assessed TX T0 No evidence of primary tumor T0 Tis Carcinoma in situ: intraepithelial or invasion of lamina propria* Tis T1 Tumor invades submucosa T1 T2 Tumor invades muscularis propria T2 T3 Tumor invades through the muscularis propria into pericolorectal tissues T3 T4a Tumor penetrates to the surface of the visceral peritoneum** T4a T4b Tumor directly invades or is adherent to other organs or structures^,** T4b *Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa. ^Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (for example, invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retro-peritoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall; or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix or vagina). **Tumor that is adherent to other organs or structures, grossly, is classified cT4b. -

EPITHELIAL TISSUE Or EPITHELIUM • the Basic Tissue of the Body
13.11.2014 Epithelium Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow EPITHELIAL TISSUE or EPITHELIUM • The basic tissue of the body. • Cells are arranged as continuous sheets. • Single or multiple layers. • Cells are held tightly together by cell junctions. • Free surface • Basal surface adheres to basal lamina or basement membrane. • Avascular but supplied by nerves. • Has high capability to regenerate. Embryological aspect • Epithelia are derived from all the 3 germ layers: • Ectoderm- Epithelium of skin • Endoderm- Epithelium of gut • Mesoderm- Epithelium of pericardial, peritoneal and pleural cavities Functions – Protection – Absorption – Barrier – Excretion – Secretory – Function as sensory surfaces Classification According to shape, arrangement and the specialization of their free surface: • Simple • Stratified • Pseudostratified • Transitional Simple epithelium Simple Squamous Epithelium • Single layered • Flat cells • On surface view, like floor tiles • Elevated nuclei Squamous • Examples: cell - Lung alveoli Nucleus - Parietal layer of Bowman’s capsule of kidney Basement - Inner aspect of membrane tympanic membrane Function: Rapid transport of - Mesothelium substances, secretion of fluid, - Endothelium diffusion of gases and osmosis Simple Squamous Epithelium Simple Cuboidal Epithelium • Single layer of cuboidal shaped cells • On surface view, cells look like mosaic (hexagonal) • Examples: -Thyroid follicles -Tubules of nephrons - Pigmented layer of retina - Germinal layer of ovary - Inner layer of -

Formation of Primary Cilia in the Renal Epithelium Is Regulated by the Von Hippel-Lindau Tumor Suppressor Protein
Fast Track Formation of Primary Cilia in the Renal Epithelium Is Regulated by the von Hippel-Lindau Tumor Suppressor Protein Miguel A. Esteban, Sarah K. Harten, Maxine G. Tran, and Patrick H. Maxwell Renal Laboratory, Imperial College London, Hammersmith Campus, London, United Kingdom Growing evidence points to defects in the primary cilium as a critical mechanism underlying renal cyst development. Inactivation of the VHL gene is responsible for the autosomal dominant condition von Hippel-Lindau (VHL) disease and is implicated in most sporadic clear cell renal carcinomas. Manifestations of VHL disease include cysts in several organs, particularly in the kidney. Here it is shown that VHL inactivation is associated with abrogation of the primary cilium in renal cysts of patients with VHL disease and in VHL-defective cell lines. Complementation of VHL-defective clear cell renal carcinoma cell lines with wild-type VHL restored primary cilia. Moreover, it is shown that the effects of VHL on the primary cilium are mediated substantially via hypoxia-inducible factor. The effect of VHL status on the primary cilium provides a potential mechanism for renal cyst development in VHL disease and may help in the understanding of how VHL acts as a tumor suppressor. J Am Soc Nephrol 17: 1801–1806, 2006. doi: 10.1681/ASN.2006020181 any different hereditary conditions are associated re-expression of VHL in cell lines that are derived from CCRCC with development of renal cysts, often with other suppresses their tumorigenicity in nude mice (11). In view of M clinical manifestations. These include autosomal the proposed role of the primary cilium in other kidney cystic dominant polycystic kidney disease, Bardet-Biedl syndrome, diseases, we hypothesized that the VHL protein (pVHL) may nephronophthisis, and oral-facial-digital type 1 syndrome. -

The Mesocolon a Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization
ORIGINAL ARTICLE The Mesocolon A Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization Kevin Culligan, MRCS,∗ Stewart Walsh, FRCSEd,∗ Colum Dunne, PhD,∗ Michael Walsh, PhD,† Siobhan Ryan, MB,‡ Fabio Quondamatteo, MD,‡ Peter Dockery, PhD,§ and J. Calvin Coffey, FRCSI∗¶ uring fetal development, the dorsal mesentery suspends the en- Background: Colonic mobilization requires separation of mesocolon from tire gastrointestinal tract from the posterior abdominal wall. The underlying fascia. Despite the surgical importance of planes formed by these D mesocolon is the adult remnant of that part of the dorsal mesentery structures, no study has formally characterized their microscopic features. associated with the colon.1 In the adult human, the transverse and The aim of this study was to determine the histological and electron micro- lateral sigmoid portions of the mesocolon are mobile whereas the as- scopic appearance of mesocolon, fascia, and retroperitoneum, prior to and cending, descending, and medial sigmoid portions are nonmobile and after colonic mobilization. attached to underlying retroperitoneum.2–4 Classic anatomic teaching Methods: In 24 cadavers, samples were taken from right, transverse, de- maintains that the ascending and descending mesocolon “disappear” scending, and sigmoid mesocolon. In 12 cadavers, specimens were stained during embryogenesis.5,6 In keeping with this, the identification of a with hematoxylin and eosin (3 sections) or Masson trichrome (3 sections). In right or left mesocolon in the adult is frequently depicted as anoma- the second 12 cadavers, lymphatic channels were identified by staining im- lous rather than accepted as an anatomic norm.7 Accordingly, the munohistochemically for podoplanin. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

ABDOMINOPELVIC CAVITY and PERITONEUM Dr
ABDOMINOPELVIC CAVITY AND PERITONEUM Dr. Milton M. Sholley SUGGESTED READING: Essential Clinical Anatomy 3 rd ed. (ECA): pp. 118 and 135141 Grant's Atlas Figures listed at the end of this syllabus. OBJECTIVES:Today's lectures are designed to explain the orientation of the abdominopelvic viscera, the peritoneal cavity, and the mesenteries. LECTURE OUTLINE PART 1 I. The abdominopelvic cavity contains the organs of the digestive system, except for the oral cavity, salivary glands, pharynx, and thoracic portion of the esophagus. It also contains major systemic blood vessels (aorta and inferior vena cava), parts of the urinary system, and parts of the reproductive system. A. The space within the abdominopelvic cavity is divided into two contiguous portions: 1. Abdominal portion that portion between the thoracic diaphragm and the pelvic brim a. The lower part of the abdominal portion is also known as the false pelvis, which is the part of the pelvis between the two iliac wings and above the pelvic brim. Sagittal section drawing Frontal section drawing 2. Pelvic portion that portion between the pelvic brim and the pelvic diaphragm a. The pelvic portion of the abdominopelvic cavity is also known as the true pelvis. B. Walls of the abdominopelvic cavity include: 1. The thoracic diaphragm (or just “diaphragm”) located superiorly and posterosuperiorly (recall the domeshape of the diaphragm) 2. The lower ribs located anterolaterally and posterolaterally 3. The posterior abdominal wall located posteriorly below the ribs and above the false pelvis and formed by the lumbar vertebrae along the posterior midline and by the quadratus lumborum and psoas major muscles on either side 4. -

Primary Retroperitoneal Mucinous Cystadenoma
Case Reports Primary retroperitoneal mucinous cystadenoma Malak S. Abedalthagafi, MD, Patrick G. Jackson, MD, Metin Ozdemirli, MD, PhD. rimary mucinous cystadenomas of the ABSTRACT Pretroperitoneum are extremely rare tumors. Although very rare cases were reported in men and children, these tumors are found exclusively in تتضمن أورام خلف الصفاق اﻷولي: السرطان الكيسي املخاطي، women.1-3 Like most retroperitoneal tumors, they can اﻷورام املخاطية ذات احلد الفاصل، اﻷورام النادرة واملتواجدة في cause symptoms through exertion of pressure or by النساء واملتضمنة احلزام املخاطي. وحيث أن خلف الصفاق اﻷولي .obstructing adjacent organs if they are large enough ﻻ يحتوي على ظاهرة مخاطية، تبقى نظرية حدوث هذه اﻷورام .They have potential for malignant transformation غير معروفة. نستنتج أن حدوث هذه اﻷورام قد يأتي من اﻷورام There is no unanimous opinion on the genesis of املسخية، أو من املبايض الزائدة، أو من التحول املخاطي للطبقة these tumors and due to their extreme rarity, their املتوسطة خللف الصفاق. نستعرض في هذا التقرير حالة للخدام histogenesis, biological behavior, and their optimal املخاطي خلف الصفاق اﻷولي ملريضة تبلغ من العمر 44 ًعاما، والتي ,management remains at a speculative level. In this paper حضرت بسبب ورم بطني. بعد استئصال الورم بجراحة املنظار we present a case of primary retroperitoneal mucinous لم يكن هناك أية أثر لعودة الورم بعد 16 شهرا.ً الشكل املجهري cystadenoma and review the clinicopathological features, therapeutic options, and outcome in respect والتحليل للصبغات النسيجية يدعم فرضية التحول املخاطي لطبقة to the cases reported in the literature. The morphologic خلف الصفاق املتوسطة واملسبوقة بتكوين كيسي اشتمالي والتي and immunohistochemical analysis observed in this تؤدي إلى حدوث أورام خلف الصفاق املخاطية.