Malignant Pleural Mesothelioma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nasal Cavity Trachea Right Main (Primary) Bronchus Left Main (Primary) Bronchus Nostril Oral Cavity Pharynx Larynx Right Lung
Nasal cavity Oral cavity Nostril Pharynx Larynx Trachea Left main Right main (primary) (primary) bronchus bronchus Left lung Right lung Diaphragm © 2018 Pearson Education, Inc. 1 Cribriform plate of ethmoid bone Sphenoidal sinus Frontal sinus Posterior nasal aperture Nasal cavity • Nasal conchae (superior, Nasopharynx middle, and inferior) • Pharyngeal tonsil • Nasal meatuses (superior, middle, and inferior) • Opening of pharyngotympanic • Nasal vestibule tube • Nostril • Uvula Hard palate Oropharynx • Palatine tonsil Soft palate • Lingual tonsil Tongue Laryngopharynx Hyoid bone Larynx Esophagus • Epiglottis • Thyroid cartilage Trachea • Vocal fold • Cricoid cartilage (b) Detailed anatomy of the upper respiratory tract © 2018 Pearson Education, Inc. 2 Pharynx • Nasopharynx • Oropharynx • Laryngopharynx (a) Regions of the pharynx © 2018 Pearson Education, Inc. 3 Posterior Mucosa Esophagus Submucosa Trachealis Lumen of Seromucous muscle trachea gland in submucosa Hyaline cartilage Adventitia (a) Anterior © 2018 Pearson Education, Inc. 4 Intercostal muscle Rib Parietal pleura Lung Pleural cavity Trachea Visceral pleura Thymus Apex of lung Left superior lobe Right superior lobe Oblique Horizontal fissure fissure Right middle lobe Left inferior lobe Oblique fissure Right inferior lobe Heart (in pericardial cavity of mediastinum) Diaphragm Base of lung (a) Anterior view. The lungs flank mediastinal structures laterally. © 2018 Pearson Education, Inc. 5 Posterior Vertebra Esophagus (in posterior mediastinum) Root of lung at hilum Right lung • Left main bronchus Parietal pleura • Left pulmonary artery • Left pulmonary vein Visceral pleura Pleural cavity Left lung Thoracic wall Pulmonary trunk Pericardial membranes Heart (in mediastinum) Sternum Anterior mediastinum Anterior (b) Transverse section through the thorax, viewed from above © 2018 Pearson Education, Inc. 6 Alveolar duct Alveoli Respiratory bronchioles Alveolar duct Terminal bronchiole Alveolar sac (a) Diagrammatic view of respiratory bronchioles, alveolar ducts, and alveoli © 2018 Pearson Education, Inc. -

Penetrating Injuries of the Pleural Cavity
Thorax: first published as 10.1136/thx.39.10.789 on 1 October 1984. Downloaded from Thorax 1984;39: 789-793 Penetrating injuries of the pleural cavity DAVID JJ MUCKART, FRED M LUVUNO, LYNNE W BAKER From the Department ofSurgery, King Edward VIII Hospital, Durban, South Africa ABSTRACT Two hundred and fifty one cases of penetrating wounds of the chest were studied prospectively. Clinical evidence is presented to show that: (1) basal intercostal drains are ade- quate to remove both air and fluid from within the pleural cavity; (2) frequent chest radio- graphs are unnecessary and intercostal drains may be removed on clinical grounds alone; (3) long term antibiotic prophylaxis is unnecessary; (4) eight per cent of those undergoing initial observation will develop a delayed haemothorax or pneumothorax of sufficient size to require drainage; (5) subcutaneous emphysema is of no prognostic significance in the symptom- less patient with minimal intrapleural damage on admission; and (6) outpatient follow up is not required. Intercostal tube drainage after penetrating chest absence of the following was documented: trauma not affecting the heart or great vessels has (1) shock as defined by a systolic blood pressure of been the standard method of treatment for many less than 100 mm Hg and a pulse rate greater than years at the King Edward VIII Hospital in Durban. 100 beats/min; (2) multiple stab wounds; Several questions concerning this approach, how- (3) severe associated head, limb or abdominal ever, remain unanswered. The use of serial chest injury; (4) respiratory distress; (5) subcutaneous radiographs and long term antibiotics, the outcome emphysema. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Progression of Idiopathic Pulmonary Fibrosis: Lessons from Asymmetrical
Interstitial lung disease Thorax: first published as 10.1136/thx.2010.137190 on 29 September 2010. Downloaded from Progression of idiopathic pulmonary fibrosis: lessons from asymmetrical disease Colas Tcherakian,1 Vincent Cottin,2 Pierre-Yves Brillet,3 Olivia Freynet,1 Nicolas Naggara,3 Zohra Carton,1 Jean-Franc¸ois Cordier,2 Michel Brauner,3 Dominique Valeyre,1 Hilario Nunes1 See Editorial, p 183 ABSTRACT decline and more of a step-like process, with periods 1Universite´ Paris 13, UPRES EA Background In idiopathic pulmonary fibrosis (IPF) the of relative steadiness punctuated by episodes of 2363, Assistance distribution and spatial-temporal progression of fibrotic acute exacerbations (AE)dthat is, acute respiratory Publique-Hoˆpitaux de Paris, changes may be influenced by general or locoregional decompensations with no identifiable cause.2 These Hoˆpital Avicenne, Service de conditions. From this perspective, patients with events may result in the majority of deaths related Pneumologie, Bobigny, France 2 2Hospices Civils de Lyon, asymmetrical disease (AIPF) may be unique. to IPF. Service de Pneumologie et Methods This retrospective study included 32 patients In IPF the distribution of fibrotic changes Centre de Re´fe´rence des (26 men, mean6SD age 6967 years) with AIPF, as between the two lungs and their spatial-temporal Maladies Pulmonaires Rares, defined by an asymmetry ratio (most affected e least progression are unknown. These may be influenced Hoˆpital Louis Pradel, Universite´ affected fibrosis score)/(most affected + least affected by various general or locoregional conditions which Lyon 1, UMR 754, Lyon (Bron), > France fibrosis score) 0.2. The global fibrosis score was the could be shared by a gradual decline and AE or be 3Universite´ Paris 13, UPRES EA average of the right and left scores. -

Lung Development
12. LUNG DEVELOPMENT Peter Rothstein, MD [email protected] RECOMMENDED READING: Larsen, Human Embryology, 3rd edition, pp 143-155. Additional sources: 1. DiFiore JW, Wilson JM. Lung development. Seminars in Pediatric Surgery 3:221-32, 1994. 2. Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J. Appl. Physiol. 81:209-24, 1996. 3. Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory distress syndrome with continuous positive airway pressure. N. Eng. J. Med. 284:1333-40, 1971. This paper describes how a simple clinical observation led to a tremendous breakthrough in the treatment of neonatal respiratory distress syndrome. 4. Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N. Eng. J Med 347:2141-48, 2002. LEARNING OBJECTIVES: You should be able to: 1. Discuss the growth and functional development of the respiratory system. 2. Discuss the stages of lung development and the major events of each stage. 3. Describe the physical and biochemical requirements for alveolar development and function. 4. Identify the developmental causes of neonatal respiratory failure, tracheoesophageal fistula and diaphragmatic hernia. GLOSSARY: Surfactant – Macromolecular complex of phospholipids and hydrophobic proteins present in alveoli that decreases surface tension and prevents alveolar collapse during exhalation. Largest lipid components are phosphatidylcholine (lecithin) and phosphatidylglycerol Type I cell (pneumocyte) – found in the airways -

Mesothelium and Malignant Mesothelioma
Journal of Developmental Biology Review Mesothelium and Malignant Mesothelioma Emilye Hiriart, Raymond Deepe and Andy Wessels * Department of Regenerative Medicine and Cell Biology, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC 29425, USA; [email protected] (E.H.); [email protected] (R.D.) * Correspondence: [email protected]; Tel.: +1-843-792-8183 Received: 4 March 2019; Accepted: 5 April 2019; Published: 8 April 2019 Abstract: The mesothelium is an epithelial structure derived from the embryonic mesoderm. It plays an important role in the development of a number of different organs, including the heart, lungs, and intestines. In this publication, we discuss aspects of the development of the mesothelium, where mesothelial structures can be found, and review molecular and cellular characteristics associated with the mesothelium. Furthermore, we discuss the involvement of the mesothelium in a number of disease conditions, in particular in the pathogenesis of mesotheliomas with an emphasis on malignant pleural mesothelioma (MPM)—a primary cancer developing in the pleural cavity. Keywords: mesothelium; development; malignant; mesothelioma; cancer 1. Introduction Malignant mesothelioma is a neoplasm that originates from mesothelial cells lining the body cavities, including the pleura, peritoneum, pericardium, and tunica vaginalis. The majority of malignant mesothelioma cases are mesotheliomas that develop in the pleural cavity. They are known as malignant pleural mesothelioma (MPM) and comprise 70–90% of all reported cases of malignant mesothelioma [1,2]. The other cases typically arise in the peritoneum [3], while the pericardium is rarely affected [4]. In this review we will briefly discuss the origin of the mesothelial structures, provide a succinct overview of molecular mechanisms involved in their development, and address aspects of the etiology and pathogenesis of mesotheliomas. -

ICD-9-CM Procedure Version 23
Procedure Code Set General Equivalence Mappings ICD-10-PCS to ICD-9-CM and ICD-9-CM to ICD-10-PCS 2008 Version Documentation and User’s Guide Preface Purpose and Audience This document accompanies the 2008 update of the Centers for Medicare and Medicaid Studies (CMS) public domain code reference mappings of the ICD-10 Procedure Code System (ICD-10- PCS) and the International Classification of Diseases 9th Revision (ICD-9-CM) Volume 3. The purpose of this document is to give readers the information they need to understand the structure and relationships contained in the mappings so they can use the information correctly. The intended audience includes but is not limited to professionals working in health information, medical research and informatics. General interest readers may find section 1 useful. Those who may benefit from the material in both sections 1 and 2 include clinical and health information professionals who plan to directly use the mappings in their work. Software engineers and IT professionals interested in the details of the file format will find this information in Appendix A. Document Overview For readability, ICD-9-CM is abbreviated “I-9,” and ICD-10-PCS is abbreviated “PCS.” The network of relationships between the two code sets described herein is named the General Equivalence Mappings (GEMs). • Section 1 is a general interest discussion of mapping as it pertains to the GEMs. It includes a discussion of the difficulties inherent in linking two coding systems of different design and structure. The specific conventions and terms employed in the GEMs are discussed in more detail. -

The Pleural Interface
Thorax 1985;40:1-8 Thorax: first published as 10.1136/thx.40.1.1 on 1 January 1985. Downloaded from Editorial The pleural interface The pleural interface is a remarkable mechanical ised by the permeability of both pleural mem- coupling in the transmission of most of the work of branes.4 breathing from the chest wall and diaphragm to the To take gases first, the reason that the pleural lungs. It is remarkable in that the coupling is under interface is kept gas free was first realised by Rist tension and yet there is no adhesive force between and Strohl,5 who pointed out that the total tension of the pleurae, so that the two surfaces remain in close gases dissolved in venous blood is about 73 cm H2O apposition and able to slide over each other easily (54 mm Hg, 7*2 kPa) below that in arterial blood. If with no tendency for fluid or gases in adjacent tis- arterial blood is equilibrated with alveolar air, this sues to enter the potential cavity which their separa- represents a total blood-gas tension 73 cm H2O tion would create. At least, this is the case under below atmospheric-compared with any gas at the normal conditions. The basic physical and physiolog- interface, which would have an absolute pressure ical principles operating in normal circumstances only 5 cm H,O below atmospheric. This gives a driv- clearly need to be appreciated before we attempt to ing force of 68 cm H2O for resolving any rationalise pathological conditions. In this article pneumothorax. -

Bleomycin Induces Pleural and Subpleural Fibrosis in the Presence of Carbon Particles
Eur Respir J 2010; 35: 176–185 DOI: 10.1183/09031936.00181808 CopyrightßERS Journals Ltd 2010 Bleomycin induces pleural and subpleural fibrosis in the presence of carbon particles N. Decologne*,+, G. Wettstein*,+, M. Kolb#, P. Margetts#, C. Garrido*, P. Camus*," and P. Bonniaud*," ABSTRACT: The pathological changes in idiopathic pulmonary fibrosis (IPF) typically start in AFFILIATIONS subpleural lung regions, a feature that is currently not explained. IPF, as well as bleomycin- *Institut National de la Sante´ et de la Recherche Me´dicale (INSERM) UMR induced lung fibrosis, are more common in smokers. We hypothesised that carbon particles, 866, University of Burgundy, which are major components of cigarette smoke that are transported to alveoli and pleural "Service de Pneumologie et surface, might be involved in the development of subpleural fibrosis through interaction with Re´animation Respiratoire CHU du pleural mesothelial cells. Bocage, Dijon, France. #Centre for Gene Therapeutics, Dept Carbon particles were administered to mice in combination with bleomycin through of Pathology and Molecular intratracheal and/or intrapleural injection and fibrosis was assessed using histomorphometry. Medicine, McMaster University, Carbon administered to the chest cavity caused severe pleural fibrosis in the presence of Hamilton, ON, Canada. + bleomycin, whereas bleomycin alone had no fibrogenic effect. The pleural response was Both authors contributed equally to this work. associated with progressive fibrosis in subpleural regions, similar to IPF in humans. Matrix accumulation within this area evolved through mesothelial-fibroblastoid transformation, where CORRESPONDENCE mesothelial cells acquire myofibroblast characteristics. In contrast, carbon did not exaggerate P. Bonniaud bleomycin-induced pulmonary fibrosis after combined intratracheal administration. Service de Pneumologie et Re´animation Respiratoire This represents a novel approach to induce a robust experimental model of pleural fibrosis. -

(CCSR) for ICD-10-PCS PROCEDURES, V2021.1
USER GUIDE: CLINICAL CLASSIFICATIONS SOFTWARE REFINED (CCSR) FOR ICD-10-PCS PROCEDURES, v2021.1 Issued December 2020 Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) Phone: (866) 290-HCUP (4287) Email: [email protected] Website: www.hcup-us.ahrq.gov TABLE OF CONTENTS What’s New in v2021.1 of the Clinical Classifications Software Refined (CCSR) for ICD-10-PCS Procedures? .............................................................................................................................. 1 Introduction ................................................................................................................................ 2 Comparison of the CCSR for ICD-10-PCS, the Beta Versions of the CCS for ICD-10-PCS, and the CCS for ICD-9-CM ............................................................................................................... 3 Description of the CCSR for ICD-10-PCS .................................................................................. 4 Understanding the Taxonomy of the ICD-10-PCS Procedure Codes ...................................... 4 The Structure of the CCSR for ICD-10-PCS ........................................................................... 7 General Assignment Guidelines .........................................................................................11 Using the CCSR to Trend ICD-10-PCS Across Data Years .......................................................12 Using the Downloadable CCSR Files ........................................................................................12 -

Benign Pleural Multicystic Mesothelioma: a Rare Case Report
Benign Pleural Multicystic Mesothelioma: A Rare Case Report Alireza Rezvani Shiraz University of Medical Sciences SeyedehMaryam Pishva Shiraz University of Medical Sciences Amirhossein Erfani Shiraz University of Medical Sciences Ahmad Monabati Shiraz University of Medical Sciences Bizhan Ziaian Shiraz University of Medical Sciences Mohammad Javad Fallahi Shiraz University of Medical Sciences Reza Shahriarirad ( [email protected] ) Shiraz University of Medical Sciences https://orcid.org/0000-0001-5454-495X Case report Keywords: Multicystic mesothelioma, Pleura, Lung Posted Date: February 2nd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-165790/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/7 Abstract Background: Fewer than 200 benign multicystic peritoneal mesothelioma cases were reported worldwide till 2017, while its pleural involvement has rarely been reported. Case presentation: We report a 70-year-old man who presented with three months history of chronic cough. Surgical resection was performed, and the pathology conrmed benign multicystic pleural mesothelioma. The patient underwent right lateral thoracotomy, wedges resection of the right upper lobe, and parietal pleurectomy and was discharged with an uneventful postop course. Conclusion: Based on published literature to date, this is the second reported case of pleural involvement of this disease. 1. Introduction Multicystic benign mesothelioma (MBM) is a rare benign tumor that presents with recurrent mesothelial cysts that arise from the epithelial and mesenchymal elements of mesothelial tissue. It has a meager potential of transformation to malignant mesothelioma. (1) BMPM was rst described in 1928 by Plaut, who incidentally observed loose pelvic cysts during an operation for uterine leiomyoma. -
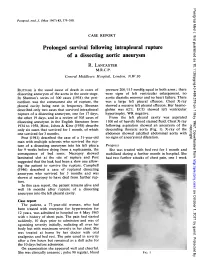
Prolonged Survival Following Intrapleural Rupture of a Dissecting Aortic Aneurysm R
Postgrad Med J: first published as 10.1136/pgmj.43.499.379 on 1 May 1967. Downloaded from Postgrad. med. J. (May 1967) 43, 379-380. CASE REPORT Prolonged survival following intrapleural rupture of a dissecting aortic aneurysm R. LANCASTER M.R.C.P. Central Middlesex Hospital, London, N.W.10 RUPTURE iS the usual cause of death in cases of pressure 200/115 mmHg equal in both arms; there dissecting aneurysm of the aorta in the acute stage. were signs of left ventricular enlargement, no In Sheenan's series of 300 cases (1934) the peri- aortic diastolic murmur and no heart failure. There cardium was the commonest site of rupture, the was a large left pleural effusion. Chest X-ray pleural cavity being next in frequency. Sheenan showed a massive left pleural effusion. Her haemo- described only two cases that survived intrapleural globin was 62%. ECG showed left ventricular rupture of a dissecting aneurysm, one for 17 days, hypertrophy. WR negative. the other 19 days, and in a review of 505 cases of From the left pleural cavity was aspirated dissecting aneurysm in the English literature from 1100 ml of heavily blood stained fluid. Chest X-ray 1934 to 1958, Hirst, Johns & Kine (1958) describe following aspiration showed an aneurysm of the copyright. only six cases that survived for 1 month, of which descending thoracic aorta (Fig. 1). X-ray of the one survived for 3 months. abdomen showed calcified abdominal aorta with Post (1941) described the case of a 51-year-old no signs of aneurysmal dilatation. man with multiple sclerosis who survived the rup- ture of a dissecting aneurysm into his left pleura Progress for 9 weeks before dying from a septicaemia, the She was treated with bed rest for 1 month and consequence of bed sores.