The Fingerprint Sourcebook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of the Skin and Its Derivatives
Development of the skin and its derivatives Resources: http://php.med.unsw.edu.au/embryology/ Larsen’s Human Embryology – Chapter 7 The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam – School of Medical Sciences, UNSW Wallace Wurth Building Room 234 – [email protected] Lecture overview Skin function and anatomy Skin origins Development of the overlying epidermis Development of epidermal appendages: Hair follicles Glands Nails Teeth Development of melanocytes Development of the Dermis Resources: http://php.med.unsw.edu.au/embryology/ Larsen’s Human Embryology – Chapter 7 The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam – School of Medical Sciences, UNSW Wallace Wurth Building Room 234 – [email protected] Skin Function and Anatomy Largest organ of our body Protects inner body from outside world (pathogens, water, sun) Thermoregulation Diverse: thick vs thin skin, scalp skin vs face skin, etc Consists of: - Overlying epidermis - Epidermal appendages: - Hair follicles, - Glands: sebaceous, sweat, apocrine, mammary - Nails - Teeth - Melanocytes - (Merkel Cells - Langerhans cells) - Dermis - Hypodermis Skin origins Trilaminar embryo Ectoderm (Neural crest) brain, spinal cord, eyes, peripheral nervous system epidermis of skin and associated structures, melanocytes, cranial connective tissues (dermis) Mesoderm musculo-skeletal system, limbs connective tissue of skin and organs urogenital system, heart, blood cells Endoderm epithelial linings of gastrointestinal and respiratory tracts Ectoderm -
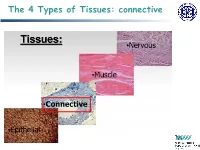
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

The Role of the Mesenchyme in 1. Patterns of Mesenchymal Cell
THE AMERICAN JOURNAL OF ANATOMY 188:121-132 (1990) The Role of the Mesenchyme in Mouse Neural Fold Elevation 1. Patterns of Mesenchymal Cell Distribution and Proliferation in Embryos Developing In Vitro JOYCE MORRIS-WIMAN AND LINDA L. BRINKLEY Department of Anatomy & Cell Biology, University of Michigan Medical School, Ann Arbor, Michigan 48109 ABSTRACT Using the computer-assisted a decrease in the density of cells residing in the central method of smoothed spatial averaging, spatial region of the mesenchyme and with a decrease in the and temporal patterns of cell distribution and mi- concentration of hyaluronate (HA) in this region (Mor- totic activity were analyzed in the cranial mesen- ris-Wiman and Brinkley, 1989). The decrease observed chyme underlying the mesencephalic neural folds in mesenchymal cell density during elevation could re- sult from a decrease in mitotic rate, from an increase in of mouse embryos maintained in roller tube cul- cell death, or from the expanion of the extracellular ture. Total cell density increased in central and matrix of the cranial mesenchyme displacing cells from medial mesenchymal regions after 12 hr in cul- this region. The glycosaminoglycan hyaluronate is the ture, decreased after 18 hr, and showed a further major component of this extracellular matrix (Solursh decrease after 24 hr when the neural folds of the and Morriss, 1977; Morriss and Solursh, 1978a,b;Hei- embryos had elevated, converged, and were fus- fetz et al., 1980). In biological and experimental sys- ing or fused. Mitotic activity, as measured by the tems, it is capable of a high degree of hydration with a ratio of 3H-thymidine-labeled cells to unlabeled concomitant increase in volume (Comper and Laurent, cells, was highest in the central mesenchyme at all 1978). -

Role of Extracellular Matrix in Regulating Embryonic Epithelial-Mesenchymal Transition
BioMol Concepts, Vol. 3 (2012), pp. 333–344 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/bmc-2011-0065 Review Role of extracellular matrix in regulating embryonic epithelial-mesenchymal transition Francesca Zito considerable amount of data about its basic mechanisms. Istituto di Biomedicina e Immunologia Molecolare From a clinical viewpoint, the results obtained so far offer ‘ Alberto Monroy ’ , Consiglio Nazionale delle Ricerche, a great promise for the development of new therapies. At Via Ugo La Malfa 153, I-90146 Palermo , Italy the same time, they advance our knowledge about essential developmental processes. e-mail: [email protected] Although EMT is a widely studied topic, it is likely that not all aspects of this process have been elucidated so far. Considerable knowledge has been accumulated regarding cell Abstract morphological changes, and the regulation of several genes and various signaling pathways have been partially under- Embryogenesis and morphogenesis are characterized by stood. Still, little is known about the role of the extracellular complex cell rearrangements and movements which require matrix (ECM) as a cell substrate during EMT, and cell motility appropriate interactions of cells with the surrounding extracel- is probably the least understood aspect of the whole process. lular matrix (ECM) by means of specifi c membrane receptors. Many events in developing embryo are associated with Interest in the identifi cation and purifi cation of ECM com- cell migration, which consists of the coordinated move- ponents, as well as in conducting functional studies of them, ments of several different cells over short or long distances including their ligands and other molecules involved in cell- throughout the embryo. -

Metamorphic Changes of Dermis in Skin of Frog Larvae Exposed to Thyroxine’
DEVELOPMENTAL BIOLOGY, 7, 244-254 (1963) Metamorphic Changes of Dermis in Skin of Frog Larvae Exposed to Thyroxine’ NORMAN E. KEMP Department of Zoology, The University of Michigan, Ann Arbor, Michigan Accepted October 22, 1962 INTRODUCTION Skin in the tadpole of anuran larvae prior to metamorphosis is composed of two layers of epidermal cells (Leeson and Threadgold, 1961; Chapman and Dawson, 1961; Edds and Sweeny, 1960, 1962), a laminated basement lamella, and a layer of dermal mesenchyme cells apposed to the underside of the basement lamella. An adepidermal space and membrane (Salpeter and Singer, 1959, 1960) separate epi- dermis from the dermal basement lamella. Weiss and Ferris (1954) first observed that mesenchyme cells migrate into the basement lamella as metamorphosis begins. Invasion of the lamella has been reported to begin about Taylor-Kollros stage XI or XII (Kemp, 1961b) and to proceed until mesenchyme cells move entirely through the lamella and cause it to become detached from the adepidermal mem- brane at stages XV-XVI. Subsequently a population of cells consti- tuting the stratum spongiosum develops beneath the adepiderma1 membrane and the basement lamella becomes the deeper-lying stra- tum compacturn or “dermal lamella” of the adult dermis. New poly- merization of collagenous fibers and ground substance results in deposition of the adult basement membrane between the adepidermal membrane and the stratum spongiosum. Generalized metamorphic changes can be induced in amphibian larvae by exposing them to thyroxine or other thyroid hormones (Kollros, 1959; Pitt-Rivers and Tata, 1959). Local metamorphic changes can be induced by implanting pellets of thyroxine mixed with ‘This investigation was supported by grants from the United States Public Health Service (RG 5867-C>), the National Science Foundation (G-19447), and the Michigan Memorial-Phoenix Project (no. -

Chondrogenesis, Studied with the Electron Microscope
CHONDROGENESIS, STUDIED WITH THE ELECTRON MICROSCOPE GABRIEL C. GODMAN, M.1)., and KEITH R. PORTER, Ph.I). From The Rockefeller Institute. Dr. Godman's present address is Department of Microbiology, Columbia University, New York ABSTRACT The role of the cells in the fabrication of a connective tissue matrix, and the structural modifications which accompany cytodifferentiation have been investigated in developing epiphyseal cartilage of fetal rat by means of electron microscopy. Differentiation of the pre- chondral mesenchymal cells to chondroblasts is marked by the acquisition of an extensive endoplasmic reticulum, enlargement and concentration of the Golgi apparatus, the ap- pearance of membrane-bounded cytoplasmic inclusions, and the formation of specialized foci of increased density in the cell cortex. These modifications are related to the secretion of the cartilage matrix. The matrix of young hyaline cartilage consists of groups of rela- tively short, straight, banded collagen fibrils of 10 to 20 m# and a dense granular component embedded in an amorphous ground substance of moderate electron density. It is postu- lated that the first phase of fibrillogenesis takes place at the cell cortex in dense bands or striae within the ectoplasm subjacent to the cell membrane. These can be resolved into sheaves of "primary" fibrils of about 7 to 10 m#. They are supposedly shed (by excortica- tion) into the matrix space between the separating chondroblasts, where they may serve as "cores" of the definitive matrix fibrils. The diameter of the fibrils may subsequently increase up to threefold, presumably by incorporation of "soluble" or tropocollagen units from the ground substance. The chondroblast also discharges into the matrix the electron- dense amorphous or granular contents of vesicles derived from the Golgi apparatus, and the mixed contents of large vacuoles or blebs bounded by distinctive double membranes. -

Intermuscular Tendons Are Essential for the Development of Vertebrate Stomach. Ludovic Le Guen, Cécile Notarnicola, Pascal De Santa Barbara
Intermuscular tendons are essential for the development of vertebrate stomach. Ludovic Le Guen, Cécile Notarnicola, Pascal de Santa Barbara To cite this version: Ludovic Le Guen, Cécile Notarnicola, Pascal de Santa Barbara. Intermuscular tendons are essential for the development of vertebrate stomach.. Development (Cambridge, England), Company of Biologists, 2009, 136, pp.791-801. 10.1242/dev.029942. inserm-00359629 HAL Id: inserm-00359629 https://www.hal.inserm.fr/inserm-00359629 Submitted on 25 Nov 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Intermuscular tendons are essential for the development of vertebrate stomach Le Guen Ludovic , Notarnicola Cécile , De Santa Barbara Pascal * Muscle et pathologies INSERM : ERI25 , IFR3 , Université Montpellier I : EA4202 , FR * Correspondence should be adressed to: Pascal De Santa Barbara <[email protected] > Abstract SUMMARY Gastrointestinal motility is ensured by the correct coordination of the enteric nervous system and the visceral smooth muscle cells (SMC), and defective development of SMCs result in gut malformations and intestinal obstructions. In order to identify the molecular mechanisms that control the differentiation of the visceral mesenchyme into SMCs in the vertebrate stomach, we developed microarrays to analyze the gene expression profiles of undifferentiated and differentiated avian stomachs. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

Invited Reviewfibroblasts and Myofibroblasts of the Intestinal
Differentiation 92 (2016) 116–131 Contents lists available at ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff Invited review Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease Manolis Roulis a, Richard A. Flavell a,b,n a Department of Immunobiology, Yale University School of Medicine, New Haven, CT 06520, USA b Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06520, USA article info abstract Article history: In this Review we summarize our current understanding of the biology of mesenchymal cells of the Received 24 February 2016 intestinal lamina propria focusing mainly on fibroblasts and myofibroblasts. The topics covered include Received in revised form 1) the embryonic origin of mesenchymal cells of the intestinal lamina propria and their heterogeneity in 19 April 2016 adults, 2) the role of the mesenchyme in intestinal development, 3) the physiological function of fi- Accepted 3 May 2016 broblasts and myofibroblasts in adults as part of the intestinal stem cell niche and the mucosal immune Available online 7 May 2016 system and 4) the involvement of fibroblasts and myofibroblasts in epithelial homeostasis upon injury Keywords: and in the pathogenesis of diseases such as Inflammatory Bowel Diseases, fibrosis and cancer. We em- Lamina propria mesenchyme phasize studies addressing the function of intestinal mesenchymal cells in vivo, and also discuss major Fibroblast open questions and current challenges in this field. fi Myo broblast & 2016 International Society of Differentiation. Published by Elsevier B.V. All rights reserved. Stromal cells Contents 1. Introduction........................................................................................................ 117 2. Architecture of the intestinal mesenchyme . 117 3. -

Development and Differentiation of Dermal Cells in Man
0022·202X/ 78/7101·0002$02.00/0 THE J OURNAL OF INVESTIGATI VE DERMATOLOGY, 71:2-8,1978 Vol. 71 , No. 1 Copyright © 1978 by The Williams & Wilkins Co. Printed in U.S.A. REPORTS Development and Differentiation of Dermal Cells in Man AODAN S. BREATHNACH, M.D. Department of Anatomy, St. Mary's Hospital M edical School, University of London, London, England Development and differentiation of the single free cells intrinsic differentiation of individual cells is influenced by in of mesenchyme and dermis of human embryos and fe tercellular communications, the transfer of macromolecules, tuses from week 6 to term is described. From week 6 to and the changing character of the environment provided by the week 14, three cell types are present: stellate general extracellular matrix. These are matters of fundamental func mesenchymal cells with long processes, phagocytic mac tional importance in development [1-8], but it is not my purpose rophages of probable yolk-sac .origin, and a granule to review them here. They are largely studied in in vitro secretory type of cell, which could be either a melano systems, and I am not in a position to contribute further to blast or a mast stem cell. From week 14 to week 21, their eluCidation from my ultrastructural observations on hu fibroblasts are numerous and active, and perineurial man embryos. Nevertheless, they must constantly be kept in cells, pericytes, melanoblasts, mast cells, and Merkel mind in situations like the present, in which a static morpho cells can be individually identified. There is also present logical approach is the only feasible one. -

The Significance of Reciprocal Induction Relations Between the Derivates of Nephrogenic Mesoderm
Rom J Leg Med [22] 199-208 [2014] DOI: 10.4323/rjlm.2014.199 © 2014 Romanian Society of Legal Medicine The significance of reciprocal induction relations between the derivates of nephrogenic mesoderm. Implications in pathology and regeneration Gheorghe S. Dragoi1,*, Petru Razvan Melinte2, Ileana Dinca2, Elena Patrascu2, Serban A. Dragoi3 _________________________________________________________________________________________ Abstract: Functional capacity of renal excretory biosystem is assured by remarkable complex structures: glomerular basal membrane for ultrafiltration of blood plasma, tubular system of the nephron for resorption-secretion processes and juxtaglomerular complex for self-balancing.The purpose of the paper is to draw the attention of histopathologists over the phenotypic transformation of metanephrogen mesenchyme and over the relations between derivates of this mesenchyme in the ontogenesis process, with implications in pathology and regeneration. Authors conducted this study and, by classical microanatomic methods, they highlighted the structural heterogeneity of renal cortex, the phenotypic variability of glomerular capillary network, the spatial distribution and the relations of intraglomerular mesangial matrix Based on personal observations, authors notice the fact that only a proper acknowledgement of reciprocal induction relations consequences between ureteric bud emerged from mesonephros and metanephrogenic blastema allows the understanding of renal parenchyma structural particularities and the determinant factors -

Distinct Signaling and Transcriptional Pathways Regulate Peri-Weaning Development and Cold-Induced Recruitment of Beige Adipocytes
Distinct signaling and transcriptional pathways regulate peri-weaning development and cold-induced recruitment of beige adipocytes Yixuan Wua, Melissa A. Kinnebrewa,b, Vassily I. Kutyavina, and Ajay Chawlaa,c,d,1,2 aCardiovascular Research Institute, University of California, San Francisco, CA 94143-0795; bDepartment of Dermatology, University of California, San Francisco, CA 94115; cDepartment of Physiology, University of California, San Francisco, CA 94143-0795; and dDepartment of Medicine, University of California, San Francisco, CA 94143-0795 Edited by Christian Wolfrum, Swiss Federal Institute of Technology in Zurich, Zurich Schwerzenbach, Switzerland, and accepted by Editorial Board Member David J. Mangelsdorf February 6, 2020 (received for review November 20, 2019) Adipose tissue provides a defense against starvation and environ- Although less well appreciated, iWAT also undergoes beige mental cold. These dichotomous functions are performed by three adipogenesis during the peri-weaning period (7–12). However, distinct cell types: energy-storing white adipocytes, and thermogenic the importance of sympathetic stimulation in acquisition of this beige and brown adipocytes. Previous studies have demonstrated phenotype remains controversial. For example, Contreras et al. that exposure to environmental cold stimulates the recruitment of demonstrated that denervation of iWAT impairs commitment to beige adipocytes in the white adipose tissue (WAT) of mice and the beige adipocyte lineage, whereas Chabowska-Kita et al. + humans, a process that has been extensively investigated. However, reported presence of UCP1 cells in the iWAT of thermoneutral beige adipose tissue also develops during the peri-weaning period in mice that presumably lacked sympathetic stimulation (7, 8). mice, a developmental program that remains poorly understood.